Abstract
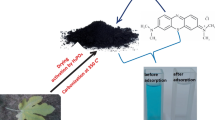
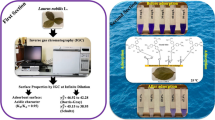
Java plum leaves ash (JLA) was prepared by calcination (400 °C) to remove cationic dyes Methylene blue dye (MBD), Acridine orange dye (AOD), and Crystal violet dye (CVD) from aqueous solutions. Multiple physiochemical and microanalytical methods, including SEM, EDX, FTIR, TGA and XRD, were used to characterize JLA. This research aims to investigate the viability of JLA as a novel adsorbent and the mechanism of its adsorption for cationic dyes. In addition, the effects of solution pH, various dye concentrations, adsorbent dosages, stirring speed, and adsorption time on JLA adsorption performance are studied. The adsorption analyses showed that the maximum adsorption capacities of MBD, AOD, and CVD are 75.87, 110.37, and 92.33 mg/g, respectively. This is much greater than other adsorbates described in the literature, suggesting the viability of JLA as a novel adsorbent within 20–60 min of contact time from an initial 100 mg/L dye solution with a dosage of 0.1 g/ml adsorbent at pH 6 and room temperature. The Langmuir isotherm, Freundlich isotherm, and Temkin isotherm models most accurately represent adsorption behaviour.



















Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to privacy concerns but may be available from the corresponding author upon reasonable request.
Change history
01 September 2023
The original online version of this article was revised: to update the university of corresponding author.
Abbreviations
- AOD:
-
Acridine orange dye
- CVD:
-
Crystal violet dye
- DTA:
-
Differential thermal analysis
- DTG:
-
Derivative thermogravimetry
- EDX:
-
Energy dispersive X-ray spectroscopy
- FTIR:
-
Fourier transform infrared spectroscopy
- JLA:
-
Java plum leaves ash
- MBD:
-
Methylene blue dye
- SEM:
-
Scanning electron microscope
- TGA:
-
Thermogravimetric analysis
- XRD:
-
X-ray diffraction
References
Alghamdi WM, El Mannoubi I (2021) Investigation of seeds and peels of citrullus colocynthis as efficient natural adsorbent for methylene blue dye. Processes 9:1279. https://doi.org/10.3390/pr9081279
Azoulay K, Bencheikh I, Moufti A et al (2020) Comparative study between static and dynamic adsorption efficiency of dyes by the mixture of palm waste using the central composite design. Chem Data Collect 27:100385. https://doi.org/10.1016/j.cdc.2020.100385
Bello OS, Adegoke KA, Fagbenro SO, Lameed OS (2019a) Functionalized coconut husks for rhodamine-B dye sequestration. Appl Water Sci 9:189. https://doi.org/10.1007/s13201-019-1051-4
Bello OS, Adegoke KA, Sarumi OO, Lameed OS (2019b) Functionalized locust bean pod (Parkia biglobosa) activated carbon for Rhodamine B dye removal. Heliyon 5:e02323. https://doi.org/10.1016/j.heliyon.2019.e02323
Bello OS, Alabi EO, Adegoke KA et al (2020) Rhodamine B dye sequestration using Gmelina aborea leaf powder. Heliyon 6:e02872. https://doi.org/10.1016/j.heliyon.2019.e02872
Borah L, Goswami M, Phukan P (2015) Adsorption of methylene blue and eosin yellow using porous carbon prepared from tea waste: adsorption equilibrium, kinetics and thermodynamics study. J Environ Chem Eng 3:1018–1028. https://doi.org/10.1016/j.jece.2015.02.013
Carneiro MT, Barros AZB, Morais AIS et al (2022) Application of water hyacinth biomass (Eichhornia crassipes) as an adsorbent for methylene blue dye from aqueous medium: kinetic and isothermal study. Polymers (basel) 14:2732. https://doi.org/10.3390/polym14132732
Chandrika K, Chaudhary A, Mareedu T et al (2021) Adsorptive removal of acridine orange dye by green tea/copper-activated carbon nanoparticles (Gt/Cu-AC np). Mater Today Proc 44:2283–2289. https://doi.org/10.1016/j.matpr.2020.12.391
Dali Youcef L, Belaroui LS, López-Galindo A (2019) Adsorption of a cationic methylene blue dye on an Algerian palygorskite. Appl Clay Sci 179:105145. https://doi.org/10.1016/j.clay.2019.105145
Das S, Mishra S (2020) Insight into the isotherm modelling, kinetic and thermodynamic exploration of iron adsorption from aqueous media by activated carbon developed from Limonia acidissima shell. Mater Chem Phys 245:122751. https://doi.org/10.1016/j.matchemphys.2020.122751
Deniz F, Kepekci RA (2017) Bioremoval of Malachite green from water sample by forestry waste mixture as potential biosorbent. Microchem J 132:172–178. https://doi.org/10.1016/j.microc.2017.01.015
El-Shafie AS, Hassan SS, Akther N, El-Azazy M (2021) Watermelon rinds as cost-efficient adsorbent for acridine orange: a response surface methodological approach. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-13652-9
Gayathri PV, Yesodharan S, Yesodharan EP (2019) Microwave/Persulphate assisted ZnO mediated photocatalysis (MW/PS/UV/ZnO) as an efficient advanced oxidation process for the removal of RhB dye pollutant from water. J Environ Chem Eng 7:103122. https://doi.org/10.1016/j.jece.2019.103122
Ghosh I, Kar S, Chatterjee T et al (2021) Removal of methylene blue from aqueous solution using Lathyrus sativus husk: adsorption study, MPR and ANN modelling. Process Saf Environ Prot 149:345–361. https://doi.org/10.1016/j.psep.2020.11.003
Hassan MM, Carr CM (2018) A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 209:201–219. https://doi.org/10.1016/j.chemosphere.2018.06.043
Hemmati F, Norouzbeigi R, Sarbisheh F, Shayesteh H (2016) Malachite green removal using modified sphagnum peat moss as a low-cost biosorbent: Kinetic, equilibrium and thermodynamic studies. J Taiwan Inst Chem Eng 58:482–489. https://doi.org/10.1016/j.jtice.2015.07.004
Hor KY, Chee JMC, Chong MN et al (2016) Evaluation of physicochemical methods in enhancing the adsorption performance of natural zeolite as low-cost adsorbent of methylene blue dye from wastewater. J Clean Prod 118:197–209. https://doi.org/10.1016/j.jclepro.2016.01.056
Islam MdA, Ahmed MJ, Khanday WA et al (2017) Mesoporous activated coconut shell-derived hydrochar prepared via hydrothermal carbonization-NaOH activation for methylene blue adsorption. J Environ Manage 203:237–244. https://doi.org/10.1016/j.jenvman.2017.07.029
Islam MR, Rahman M, Farhad SFU, Podder J (2019) Structural, optical and photocatalysis properties of sol–gel deposited Al-doped ZnO thin films. Surf Interfaces 16:120–126. https://doi.org/10.1016/j.surfin.2019.05.007
Jawad AH, Abdulhameed AS (2020) Mesoporous Iraqi red kaolin clay as an efficient adsorbent for methylene blue dye: adsorption kinetic, isotherm and mechanism study. Surf Interfaces 18:100422. https://doi.org/10.1016/j.surfin.2019.100422
Jawad AH, Abdulhameed AS, Mastuli MS (2020a) Acid-factionalized biomass material for methylene blue dye removal: a comprehensive adsorption and mechanism study. J Taibah Univ Sci 14:305–313. https://doi.org/10.1080/16583655.2020.1736767
Jawad AH, Abdulhameed AS, Reghioua A, Yaseen ZM (2020b) Zwitterion composite chitosan-epichlorohydrin/zeolite for adsorption of methylene blue and reactive red 120 dyes. Int J Biol Macromol 163:756–765. https://doi.org/10.1016/j.ijbiomac.2020.07.014
Jawad AH, Mubarak NSA, Abdulhameed AS (2020c) Tunable Schiff’s base-cross-linked chitosan composite for the removal of reactive red 120 dye: adsorption and mechanism study. Int J Biol Macromol 142:732–741. https://doi.org/10.1016/j.ijbiomac.2019.10.014
Jawad AH, Saud Abdulhameed A, Wilson LD et al (2021) High surface area and mesoporous activated carbon from KOH-activated dragon fruit peels for methylene blue dye adsorption: optimization and mechanism study. Chin J Chem Eng 32:281–290. https://doi.org/10.1016/j.cjche.2020.09.070
Jayasantha Kumari H, Krishnamoorthy P, Arumugam TK et al (2017) An efficient removal of crystal violet dye from waste water by adsorption onto TLAC/Chitosan composite: a novel low cost adsorbent. Int J Biol Macromol 96:324–333. https://doi.org/10.1016/j.ijbiomac.2016.11.077
Jegatheesan V, Pramanik BK, Chen J et al (2016) Treatment of textile wastewater with membrane bioreactor: a critical review. Bioresour Technol 204:202–212. https://doi.org/10.1016/j.biortech.2016.01.006
Jiang X, Sun P, Xu L et al (2020) Platanus orientalis leaves based hierarchical porous carbon microspheres as high efficiency adsorbents for organic dyes removal. Chin J Chem Eng 28:254–265. https://doi.org/10.1016/j.cjche.2019.03.030
Kataria N, Garg VK (2019) Application of EDTA modified Fe3O4/sawdust carbon nanocomposites to ameliorate methylene blue and brilliant green dye laden water. Environ Res 172:43–54. https://doi.org/10.1016/j.envres.2019.02.002
Khasri A, Bello OS, Ahmad MA (2018) Mesoporous activated carbon from Pentace species sawdust via microwave-induced KOH activation: optimization and methylene blue adsorption. Res Chem Intermed 44:5737–5757. https://doi.org/10.1007/s11164-018-3452-7
Kiran S, Nosheen S, Abrar S, et al (2019) Advanced approaches for remediation of textile wastewater: a comparative study. In: Advanced Functional Textiles and Polymers. Wiley, pp 201–264
Lebron YAR, Moreira VR, de Souza Santos LV (2021) Biosorption of methylene blue and eriochrome black T onto the brown macroalgae Fucus vesiculosus : equilibrium, kinetics, thermodynamics and optimization. Environ Technol 42:279–297. https://doi.org/10.1080/09593330.2019.1626914
Meili L, Lins PVS, Costa MT et al (2019) Adsorption of methylene blue on agroindustrial wastes: experimental investigation and phenomenological modelling. Prog Biophys Mol Biol 141:60–71. https://doi.org/10.1016/j.pbiomolbio.2018.07.011
Oyekanmi AA, Ahmad A, Hossain K, Rafatullah M (2019) Statistical optimization for adsorption of Rhodamine B dye from aqueous solutions. J Mol Liq 281:48–58. https://doi.org/10.1016/j.molliq.2019.02.057
Putri KNA, Keereerak A, Chinpa W (2020) Novel cellulose-based biosorbent from lemongrass leaf combined with cellulose acetate for adsorption of crystal violet. Int J Biol Macromol 156:762–772. https://doi.org/10.1016/j.ijbiomac.2020.04.100
Rangabhashiyam S, Lata S, Balasubramanian P (2018) Biosorption characteristics of methylene blue and malachite green from simulated wastewater onto Carica papaya wood biosorbent. Surf Interfaces 10:197–215. https://doi.org/10.1016/j.surfin.2017.09.011
Saha P, Chowdhury S, Gupta S, Kumar I (2010) Insight into adsorption equilibrium, kinetics and thermodynamics of Malachite Green onto clayey soil of Indian origin. Chem Eng J 165:874–882. https://doi.org/10.1016/j.cej.2010.10.048
Santoso E, Ediati R, Kusumawati Y et al (2020) Review on recent advances of carbon based adsorbent for methylene blue removal from waste water. Mater Today Chem 16:100233. https://doi.org/10.1016/j.mtchem.2019.100233
Saratale RG, Rajesh Banu J, Shin H-S, et al. (2020) Textile industry wastewaters as major sources of environmental contamination: bioremediation approaches for its degradation and detoxification. In: Bioremediation of Industrial Waste for Environmental Safety. Springer Singapore, Singapore, pp 135–167
Saravanan P, Josephraj J, Thillainayagam BP, Ravindiran G (2023) Evaluation of the adsorptive removal of cationic dyes by greening biochar derived from agricultural bio-waste of rice husk. Biomass Convers Biorefin 13:4047–4060. https://doi.org/10.1007/s13399-021-01415-y
Shakoor S, Nasar A (2016) Removal of methylene blue dye from artificially contaminated water using citrus limetta peel waste as a very low cost adsorbent. J Taiwan Inst Chem Eng 66:154–163. https://doi.org/10.1016/j.jtice.2016.06.009
Shetti NP, Malode SJ, Malladi RS et al (2019) Electrochemical detection and degradation of textile dye Congo red at graphene oxide modified electrode. Microchem J 146:387–392. https://doi.org/10.1016/j.microc.2019.01.033
Siddiqui SH (2018) The removal of Cu2+, Ni2+ and Methylene Blue (MB) from aqueous solution using Luffa Actangula Carbon: kinetics, thermodynamic and isotherm and response methodology. Groundw Sustain Dev 6:141–149. https://doi.org/10.1016/j.gsd.2017.12.008
Singh H, Chauhan G, Jain AK, Sharma SK (2017) Adsorptive potential of agricultural wastes for removal of dyes from aqueous solutions. J Environ Chem Eng 5:122–135. https://doi.org/10.1016/j.jece.2016.11.030
Smitha T, Santhi T, Prasad AL, Manonmani S (2017) Cucumis sativus used as adsorbent for the removal of dyes from aqueous solution. Arab J Chem 10:S244–S251. https://doi.org/10.1016/j.arabjc.2012.07.030
Sri Devi V, Sudhakar B, Prasad K et al (2020) Adsorption of Congo red from aqueous solution onto Antigonon leptopus leaf powder: equilibrium and kinetic modeling. Mater Today Proc 26:3197–3206. https://doi.org/10.1016/j.matpr.2020.02.715
Tirkey P, Bhattacharya T, Chakraborty S (2018) Optimization of fluoride removal from aqueous solution using Jamun (Syzygium cumini) leaf ash. Process Saf Environ Prot 115:125–138. https://doi.org/10.1016/j.psep.2017.10.022
Vyavahare G, Jadhav P, Jadhav J et al (2019) Strategies for crystal violet dye sorption on biochar derived from mango leaves and evaluation of residual dye toxicity. J Clean Prod 207:296–305. https://doi.org/10.1016/j.jclepro.2018.09.193
Wang H, Zhao W, Chen Y, Li Y (2020) Nickel aluminum layered double oxides modified magnetic biochar from waste corncob for efficient removal of acridine orange. Bioresour Technol 315:123834. https://doi.org/10.1016/j.biortech.2020.123834
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interface Sci 209:172–184. https://doi.org/10.1016/j.cis.2014.04.002
Yang J-X, Hong G-B (2018) Adsorption behavior of modified Glossogyne tenuifolia leaves as a potential biosorbent for the removal of dyes. J Mol Liq 252:289–295. https://doi.org/10.1016/j.molliq.2017.12.142
Zaidi NAHM, Lim LBL, Usman A (2019) Enhancing adsorption of malachite green dye using base-modified Artocarpus odoratissimus leaves as adsorbents. Environ Technol Innov 13:211–223. https://doi.org/10.1016/j.eti.2018.12.002
Zeng L, Xie M, Zhang Q et al (2015) Chitosan/organic rectorite composite for the magnetic uptake of methylene blue and methyl orange. Carbohydr Polym 123:89–98. https://doi.org/10.1016/j.carbpol.2015.01.021
Zhao B, Sun X, Wang L et al (2019) Adsorption of methyl orange from aqueous solution by composite magnetic microspheres of chitosan and quaternary ammonium chitosan derivative. Chin J Chem Eng 27:1973–1980. https://doi.org/10.1016/j.cjche.2018.12.014
Acknowledgements
The authors gratefully acknowledge sample analysis support received from the Sophisticated Instrumentation Centre for Applied Research and Testing (SICART), Vallabh Vidyanagar, Gujarat and Sophisticated Analytical Instrumentation Facility (SAIF & CIL), Chandigarh, Punjab. The authors acknowledge Dr Sunil Chaki, Department of Physics, Sardar Patel University, Anand, Gujarat for providing TGA, DTA and DTG measurement facilities and fruitful discussions. One of the authors (MRP) is grateful to SHODH ScHeme of Developing High Quality Research (KCG/SHODH/2020-21), Knowledge Consortium of Gujarat, Government of Gujarat for the scholarship.
Funding
SHODH—ScHeme of Developing High-Quality Research, Knowledge Consortium of Gujarat, (Education Department, Government of Gujarat. Letter Reference no: KCG/SHODH/2020–21/ & Grant number: 202010820024.
Author information
Authors and Affiliations
Contributions
Dr. Arvnabh Mishra has given guidance & idea about this work, and he has supervised, monitored, and revised the manuscript. Dr. Manish Mishra has mentored me throughout the manuscript writing and in the collection of data. Meera R. Popaliya has collected data, designed the manuscript and written the main text, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article, and the authors also declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent to participate
Not applicable.
Consent to publiction
Not applicable.
Ethical approval
We hereby declare that this work is original and not copied from anywhere else. We approve that this work is not submitted in this language or any other language to any other journal nor it is submitted for simultaneous consideration.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: to update the university of corresponding author.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Popaliya, M.R., Mishra, M. & Mishra, A. Removal of cationic dyes onto java plum leaves ash: adsorption isotherms, kinetics, thermodynamic and characterizations. Chem. Pap. 77, 7881–7901 (2023). https://doi.org/10.1007/s11696-023-03037-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03037-2