Abstract
Haemorrhagic septicaemia (HS) is an endemic disease of bovines, occuring in most tropical regions of Asia and Africa. In the present study, the suitability of using mice to study pathogenesis of HS was assessed using mortality, mean death time and bacterial multiplication in vital organs after infection with live P multocida. Mice were infected with 105, 103 and 101 cfu of P. multocida B:2 via intranasal and subcutaneous routes along with control groups. Bacterial multiplication in lung, liver and spleen of mice were determined at 24 h interval after intranasal and subcutaneous challenge. More than 80 % of challenged mice died within 48 h of inoculation, irrespective of the dose and route of inoculation. A heavy bacterial load (up to 108 cfu) was observed in lung, liver and spleen of mice titrated at 24 h and following death of mice. Results of the present study indicate that even ten bacteria are enough to cause mortality in mice and the organism multiplies rapidly in respiratory epithelium and disseminated to other vital organs viz liver and spleen suggesting the important role of mouse model in investigating the pathogenesis and challenge studies during vaccine development.
Similar content being viewed by others
Introduction
Haemorrhagic septicaemia (HS) is a fatal systemic disease of bovines characterized by an acute, highly fatal septicaemia with high morbidity and mortality. The disease is caused by serotypes B and E strains of Pasteurella multocida in Asian and African countries respectively (De Alwis 1992). HS is endemic in most parts of tropical Asia and Africa. Although the organism does not survive outside the animal body for a long period, it can survive up to several days in moist soil and water leading to wide transmission during monsoon season causing heavy economic losses (Bankirane and De Alwis 2002).
For a successful infection, the bacterial pathogens must be able to acquire sufficient nutrients for growth and multiplication while avoiding the host immune system. If the infection is to result in disease, the pathogen might also release toxins or toxic by-products, or interact with the host in a way which results in perturbation of homeostasis (Boyce and Adler 2006). Pathogenesis of HS is not clearly understood, however, it is believed that during stress conditions bacteria multiply and septicaemia develops leading to death due to endotoxaemia (Horadagoda et al. 2001). Key virulence factors identified include capsule, lipopolysaccharides, surface adhesions, iron-regulated and iron acquisition proteins etc. However, many key virulence factors are yet to be identified, including those required for initial attachment and invasion of host cells (Harper et al. 2006).
Pathogenesis of P. multocida is a complex interaction between host specific factors and specific bacterial virulence factors, therefore, understanding the disease pathogenesis is complex and depends on the bacterial strain, the animal model and their interactions (Boyce and Adler 2006). Additionally, the early multiplication of P. multocida in various organs of the host is a very crucial step in the study of pathogenesis and development of a disease model. Therefore, the present work is aimed at to study the bacterial multiplication and dissemination of P. multocida B:2 in various vital organs of mice after inoculation through natural (intranasal) and experimental (subcutaneous) routes of infection.
Materials and methods
Bacterial strain and growth conditions
The culture of P. Multocida, P52 strain (originally obtained from Haryana Veterinary Vaccine Institute, Hisar) was purified by passaging in mice (Bain 1955). Purity of the culture was confirmed using culture characteristics, Gram’s staining and various biochemical tests viz catalase, oxidase, indole and nitrate tests. The stock culture was then prepared from the pure culture by inoculating on brain heart infusion (BHI) agar enriched with 7 % (v/v) sheep blood and incubating at 37 °C for 18 h.
Titration of the stock culture
The stock culture was titrated on petri-plates (BHI agar enriched with sheep blood) as well as 24-well plates (BHI Agar) simultaneously to ensure the accuracy of titers of the stock culture. Briefly, ten-fold serial dilutions of the culture were prepared in cold normal saline solution (NSS) by mixing 100 μl of culture in 900 μl of NSS. Volumes of 200 μl and 20 μl from various dilutions of the stock culture were transferred on petri-plates and 24-well plates respectively. The plates were incubated at 37 °C up to 48 h. The number of colonies in the last countable dilution was multiplied with the dilution factor to determine the titers of the stock culture.
Animals
Sixty Swiss albino female mice of 6 to 8-week age were procured from Disease Free Small Animal House of the University after obtaining permission from Institutional Animal Ethical Committee. Mice were housed under standard housing conditions. Sero-negativity of the mouse colony for P. Multocida was reassured in ELISA.
Animal experiments
Experiment 1
Thirty Swiss albino female mice were divided into four groups as group A (5 mice), group B (10 mice), group C (10 mice) group D (5 mice). All mice were inoculated via intranasal route. Mice from group A, B and C were inoculated with 105, 103 and 101 cfu of live P. multocida inoculums respectively, under mild inhalant anesthesia (ether) in a volume of 20 μl/mouse. The inoculums were allowed to inhale by placing it on the nostrils of the mice. Group D mice were kept as control and were inoculated with NSS.
Experiment 2
Mice were inoculated with 200 μl of inoculums per mouse via subcutaneous route, keeping the number of mice per group and dose of inoculums same as in Experiment 1.
Number of mice in group A (inoculated with high doses) and group D (control animals) used were small, mainly for humane and economic reasons. Mortality was recorded and mean death time was calculated in each group.
Collection of tissues
Tissues were collected from lung, liver and spleen of all the three groups of mice infected via intranasal and subcutaneous routes at 24 h after inoculation and following death (before 48 h). Tissues were collected aseptically in pre-weighed bottles from three mice in each group individually.
Titration of bacterial load in organs
To measure the bacterial load in different groups of mice, the tissues collected were homogenized aseptically in NSS to make 10 % suspension and tenfold serial dilution were made. These dilutions were cultured in triplicate on BHI agar in 24 well-plates by incubating at 37 °C up to 48 h. The titers were expressed as cfu per gram of tissue.
Results
Mortality and mean death time (MDT)
Percent mortality and mean death time were calculated in all the groups of mice following intranasal and subcutaneous inoculation of 105, 103 and 101 cfu of live P. multocida (Table 1). Irrespective of the dose and route of inoculation, more than 80 % of infected mice died within 48 h, whereas no mortality was observed in control groups of mice. Results of the present study indicate that as low as ten bacteria caused mortality in mice suggesting mouse highly susceptible for P. multocida infection. It was interesting to observe that the mean death time as well as percent mortality in the groups of mice inoculated with 105, 103 or 101 cfu were independent of the dose of inoculums. A trivial reason for this could be a very short multiplication time of the organism (generation time as short as 36 min) which could be argued favorably since HS is disease of per acute nature. A rapid multiplication of P. multocida in mouse has also been reported earlier with as few as 20 cfu produced septicaemia resulting in mortality within 30 h (Ramdani et al. 1990). Even in natural host, the organism multiplies rapidly causing death in as early as 18 h after onset of clinical signs.
Bacterial multiplication in organs
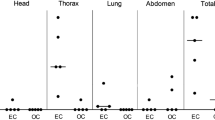
The dissemination and multiplication of bacteria in different organs was examined by titrating the organism after inoculation through intranasal and subcutaneous routes. Bacterial load was titrated in lung, liver and spleen of mice from inoculated groups of mice along with control mice. A heavy bacterial count (up to 108 cfu) was observed at 24 h in all the three organs of infected mice. Following intranasal challenge, as the dose of inoculum increased from 101 to 105 cfu, the bacterial titers also increased from 106 to 108 cfu showing some dose dependent correlation between dose of inoculum and the bacterial titers but the values were not found significant (P > 0.05, student’s t test; Table 2). However, no such dose dependent response was observed following subcutaneous challenge (Table 3). The results showed that intranasal and subcutaneous inoculation of live P. multocida allowed rapid multiplication and dissemination of bacteria to all of the tissues examined upon death.
Discussion
Haemorrhagic septicaemia (HS) is an infectious disease of bovines caused by serotypes B:2 and E:2 of Pasteurella multocida in Asian and African countries respectively. The disease is endemic in most parts of tropical Africa and Asia including India. Because of lack of a suitable animal model, only limited information is available with respect to the pathogenesis of disease. The results of the present study describe intranasal and subcutaneous infection of mice with P52 strain of P. multocida B:2. Mice are exquisitely susceptible to P. multocida with our studies showing even ten bacteria are enough to cause mortality in mice constituting a critical pathogenic dose.
In a study using the mouse model of HS, Ramdani et al. (1990) made a similar observation using the Ml404 strain of P. multocida. Intraperitoneal challenge of female BALB/c mice with as few as 20 cfu of P. multocida produced an overwhelming septicaemia in mice within 30 h indicating the suitability of mouse model in HS studies. In another study on investigating the virulence properties of two strains of P. multocida B:2, mice were challenged intraperitonealy with 101 to 103 cfu of M1404 and PBA1514 strains. Within 24 h of challenge, 10 cfu and 20 cfu of M1404 and PBA1514 strains caused mortality in 83 % of challenged mice respectively (Boyce and Adler 2000). In our earlier study using P52 strain aimed at evaluation of novel vaccines for HS, the control group of mice died within 48 h whereas mice immunized intranasally withstood challenge with as high as 108 cfu of live P. multocida (Kharb and Charan 2011). Similar results were also reviewed by Frost and Adler (2000). Results of the present study indicate that even ten bacteria are adequate to cause mortality in mice suggesting that mouse being highly sensitive and susceptible to P. multocida can serve as a useful model for pathogenesis studies and challenge studies including vaccines development against HS.
The numbers of bacteria in various organs of mice were determined at 24 h and following death. Heavy bacterial loads of 108 cfu were measured in lung, liver and spleen of infected mice at 24 h of inoculation which remained high following mortality. However, a significant dose dependent response was not observed. It might be because after attaining a threshold value of certain cytokines viz TNF-α, IL-1, IL-6 produced by endotoxin stimulated monocytes, the dose of inoculums might be of little impact. Also the over-stimulation of TNF-α may result in intravascular coagulation, hypotension and life threatening shock (Horadagoda et al. 2002).
Boyce and Adler (2000) also reported rapid multiplication of bacteria in all organs of mice until lethal levels were reached following intraperitoneal challenge with 5 × 104 cfu of PBA1514 strain of P. multocida B:2. A heavy bacterial load of 5 × 107 cfu per ml of blood and 6 × 107 cfu per total organ in liver and spleen of mice has been reported by them. In another study to investigate the pathogenesis of an isolate, a dose of 8 × 102 and 8 × 104 cfu of live P. multocida B:2 was used via intraperitoneal and intranasal routes respectively. Bacterial multiplication was measured in lung, liver, spleen and blood at 24 h intervals. Intraperitonealy inoculated mice died within 36 h with bacterial counts between 1010 and 1012 cfu at 24 h of inoculation. However, the mice inoculated intranasally survived up to 96 h with bacterial load between 104 and 105 cfu at 24 h which declined substantially (<102) at 48 h except in lungs (108) and at 72 h was found elevated between 109 and 1011 cfu in all the vital organs (Tabatabai et al. 2002). In our earlier study conducted on immunogenicity of iron regulated outer membrane proteins, bacterial titers of 107 cfu were observed in lung, liver and spleen of control mice following inoculation with 102 cfu of live P. multocida via intranasal and subcutaneous routes (Kharb and Charan 2010). In the present study the challenge route adopted was subcutaneous rather than the intraperitoneal adopted by other workers. This variation provided an opportunity to explore the possibility of use of an alternative route since during primary isolation from the disease outbreak inoculation via intraperitoneal route may be more tedious and risk prone.
Although it is not clearly understood where the organism lodges and multiplies during the early phase, taken together these studies seem to indicate that the bacteria invade the mucosal epithelial layers, as following infection through intranasal route, bacteria multiply rapidly in the respiratory tract (lungs) and disseminate to the other vital organs viz liver and spleen, as reported by other workers also (Wijewardana 1992; Tabatabai et al. 2002). A similar disease progression occurs in fowl cholera caused by serotype A of P. multocida (Rabier et al. 1997). Some studies with avian strains have shown the role of macrophages and neutrophills in uptake and survival of the organism in turkey and chicken (Harmon et al. 1992; Poermadjaja and Frost 2002). However, it still remains to be resolved, whether the multiplication first occurs in respiratory epithelium and then organism is carried to different organs or after the initial multiplication in respiratory epithelium the major multiplication occurs in different vital organs.
Although the role of different cells in dissemination of P. multocida in mouse model as well as in natural host is not well known but the magnitude of microbial multiplication and clinical signs of the disease suggest that mouse can serve as useful model for the study of pathogenesis and vaccine development.
References
Bain RVS (1955) Studies on haemorrhagic septicaemia of cattle. IV. A preliminary examination of antigens of Pasteurella multocida type I. Br Vet J 111:492
Bankirane A, De Alwis MCL (2002) Haemorrhagic septicaemia, its significance, prevention and control in Asia. Vet Med-Czech 47:234–240
Boyce JD, Adler B (2000) The capsule is a virulence determinant in the pathogenesis of Pasteurella multocida M1404 (B:2). Infect Immun 68:3463–3468
Boyce JD, Adler B (2006) How does Pasteurella multocida respond to the host environment? Curr Opin Microbiol 9:117–122
De Alwis MCL (1992) Haemorrhagic septicaemia—a general review. Br Vet J 148:99–112
Frost A, Adler B (2000) Pasteurella multocida: the elusive determinants of virulence and immunity. Vet Microbiol 72:1–2
Harmon BG, Glisson JR, Latimer KS, Nunnly JC (1992) Turkey macrophage and heterophill bactericidal activity against Pasteurella multocida. Avian Dis 36:989–991
Harper M, Boyce JD, Adler B (2006) Pasteurella multocida pathogenesis: 125 years after Pasteur. FEMS Microbiol Lett 265:1–10
Horadagoda NU, Hodgson JC, Moon GM, Wijewardana TG, Eckersall PD (2001) Role of endotoxin in the pathogenesis of haemorrhagic septicaemia in the buffalo. Microb Pathogen 30:171–178
Horadagoda NU, Hodgson JC, Moon GM, Wijewardana TG, Eckersall PD (2002) Development of a clinical syndrome resembling haemorrhagic septicaemia in the buffalo following intravenous inoculation of Pasteurella multocida serotype B:2 endotoxin and the role of tumor necrosis factor-α. Res Vet Sc 72:194–200
Kharb S, Charan S (2010) Immunogenicity of iron-regulated outer membrane proteins of Pasteurella multocida B:2 in mice model. Indian J Exp Biol 48:1181–1187
Kharb S, Charan S (2011) Mucosal immunization provides better protection than subcutaneous immunization against Pasteurella multocida (B:2) in mice preimmunized with the outer membrane proteins. Vet Res Comm 35:457–461
Poermadjaja B, Frost A (2002) Phagocytic uptake and killing of virulent and avirulent strains of Pasteurella multocida of capsular serotype A by chicken macrophages. Vet Microbiol 72:163–171
Rabier MJ, Tyler NK, Walker NJ, Hansen LM, Hirsh DC, Tablin F (1997) Pasteurella multocida enters polarized epithelial cells by interacting with host F-actin. Vet Microbiol 54:343–355
Ramdani, Dawkins HJS, Johnson RB, Spencer TL, Adler B (1990) Pasteurella multocida infections in mice with reference to haemorrhagic septicaemia in cattle and buffalo. Immunol Cell Biol 68:57–61
Tabatabai M, Liu Z, Finucane A, Parton R, Coote J (2002) Protective immunity conferred by attenuated aroA derivatives of Pasteurella multocida B:2 strains in a mouse model of haemorrhagic septicaemia. Infect Immun 70:3355–3362
Wijewardana TG (1992) Haemorrhagic septicaemia. Rev Med Microbiol 3:59–63
Acknowledgement
Authors are thankful to Haryana Veterinary Vaccine Institute, Hisar for providing bacterial strain and Institutional Animal Ethical Committee for permitting us to conduct the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The experiments were conducted after obtaining permission from Institutional Animal Ethical Committee and comply with the current laws of the country.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kharb, S., Charan, S. Mouse model of haemorrhagic septicaemia: dissemination and multiplication of Pasteurella multocida B:2 in vital organs after intranasal and subcutaneous challenge in mice. Vet Res Commun 37, 59–63 (2013). https://doi.org/10.1007/s11259-012-9547-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-012-9547-5