Abstract
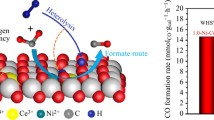
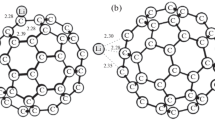
The effect of U cations on the reduction of CeO2 is studied by modeling the Ce1−xUxO2 systems with density functional theory (DFT) at the GGA + U (U = 5.0 eV) level of theory. Oxygen-vacancy formation energies (EOvac) are influenced by substituting Ce4+ cations in CeO2 by U4+ cations. The pDOS analyses indicate charge transfer mechanism from the O2p and U5f orbitals to the Ce4f orbitals upon the creation of an oxygen vacancy resulting in the reduction of three Ce4+ cations (instead of two in the un-doped system) to three Ce3+ cations. A linear correlation between EOvac and U content has been found which may explain the initially observed experimental correlation between H2 production from water on this system. The increased hydrogen production from water using mixed charged elements (such as Ce and U in this work) may be rationally designed computationally.





Similar content being viewed by others
References
Abanades S, Legal A, Cordier A, Peraudeau G, Flamant G, Julbe A (2010) Investigation of reactive cerium-based oxides for H2 production by thermochemical two-step water-splitting. J Mater Sci 45:4163–4173
Zhu X, Wang H, Wei Y, Li K, Cheng X (2011) Hydrogen and syngas production from two-step steam reforming of methane using CeO2 as oxygen carrier. J Nat Gas Chem 20:281–286
Al-Salik Y, Al-Shankiti I, Idriss H (2013) Core level spectroscopy of oxidized and reduced CexU1−xO2 materials. J. Electron Spectrosc Relat Phenom 194:66–73
Al-Shankiti I, Al-Otaibi F, Al-Salik Y, Idriss H (2013) Solar thermal hydrogen production from water over modified CeO2 materials. Top Catal 56:1129–1138
Chueh WC, Falter C, Abbott M, Scipio D, Furler P et al (2010) High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria. Science 330:1797–1801
Steinfeld A (2005) Solar thermochemical production of hydrogen—a review. Sol Energy 78:603–615
Abanades S, Flamant G (2006) Thermochemical hydrogen production from a two-step solar-driven water-splitting cycle based on cerium oxides. Sol Energy 80:1611–1623
Le Gal A, Abanades S (2012) Dopant incorporation in ceria for enhanced water-splitting activity during solar thermochemical hydrogen generation. J Phys Chem C 116:13516–13523
Meng Q-L, Lee C-I, Ishihara T, Kaneko H, Tamaura Y (2011) Reactivity of CeO2-based ceramics for solar hydrogen production via a two-step water-splitting cycle with concentrated solar energy. Int J Hydrog Energy 36:13435–13441
Yang Z, Woo TK, Hermansson K (2006) Effects of Zr doping on stoichiometric and reduced ceria: a first-principles study. J Chem Phys 124:224704
Dholabhai PP, Adams JB, Crozier P, Sharma R (2010) Oxygen vacancy migration in ceria and Pr-doped ceria: a DFT + U study. J Chem Phys. 132:094104
Andersson DA, Simak SI, Skorodumova NV, Abrikosov IA, Johansson B (2007) Redox properties of CeO2–MO2 (M = Ti, Zr, Hf, or Th) solid solutions from first principles calculations. Appl Phys Lett 90:031909
Xu W, Si R, Senanayake SD, Llorca J, Idriss H et al (2012) In situ studies of CeO2-supported Pt, Ru, and Pt-Ru alloy catalysts for the water-gas shift reaction: active phases and reaction intermediates. J Catal 291:117–126
Diagne C, Idriss H, Kiennemann A (2002) Hydrogen production by ethanol reforming over Rh/CeO2-ZrO2 catalysts. Catal Commun 3:565–571
Idriss H (2010) Surface reactions of uranium oxide powder, thin films and single crystals. Surf Sci Rep 65:67–109
Hanken BE, Stanek CR, Grønbech-Jensen N, Asta M (2011) Computational study of the energetics of charge and cation mixing in U1−xCexO2. Phys Rev B 84:085131
Hinatsu Y, Fujino T (1988) Studies on magnetic properties of UO2-CeO2 solid solutions. II. Effect of oxygen nonstoichiometry on magnetic susceptibilities of solid solutions. J Solid State Chem 76:124–130
Antonio MR, Staub U, Xue JS, Soderholm L (1996) Comparison of the cation valence and coordination in Ce2UO6 and Ce2MoO6. Chem Mater 8:2673–2680
Bera S, Mittal VK, Venkata Krishnan R, Saravanan T, Velmurugan S et al (2009) XPS analysis of UxCe1-xO2±δ and determination of oxygen to metal ratio. J Nucl Mater 393:120–125
Kresse G, Hafner J (1993) Ab initio molecular dynamics for liquid metals. Phys Rev B 47:558–561
Kresse G, Furthmuller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54:11169–11186
Blöchl PE (1994) Projector augmented-wave method. Phys Rev B 50:17953–17979
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Dudarev SL, Botton GA, Savrasov SY, Humphreys CJ, Sutton AP (1998) Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA + U study. Phys Rev B 57:1505–1509
Paier J, Penschke C, Sauer J (2013) Oxygen defects and surface chemistry of ceria: quantum chemical studies compared to experiment. Chem Rev 113:3949–3985
Lorenzelli R, Touzelin B (1980) Sur le système UO2-CeO2; étude cristallographique à haute température. J Nucl Mater 95:290–302
Magneli AK, Kihlborg L (1951) On the cerium dioxide-uranium dioxide system and uranium cerium blue. Acta Chem Scand 5:3
Frazer BC, Shirane G, Cox DE, Olsen CE (1965) Neutron-diffraction study of antiferromagnetism in UO2. Phys Rev 140:A1448–A1452
Yu J, Devanathan R, Weber WJ (2009) First-principles study of defects and phase transition in UO2. J Phys Condens Matter 21:435401
Markin TL, Street RS, Crouch EC (1970) The uranium-cerium-oxygen ternary phase diagram. J Inorg Nucl Chem 32:59–75
Dorado B, Garcia P (2013) First-principles DFT + U modeling of actinide-based alloys: application to paramagnetic phases of UO2 and (U, Pu) mixed oxides. Phys Rev 87:195139
Caciuffo R, Magnani N, Santini P, Carretta S, Amoretti G et al (2007) Anisotropic magnetic fluctuations in 3-k antiferromagnets. J Magn Magn Mater 310:1698–1702
Gryaznov D, Heifets E, Sedmidubsky D (2010) Density functional theory calculations on magnetic properties of actinide compounds. Phys Chem Chem Phys 12:12273–12278
Yun YS, Park KH, Kim HC, Kim HM (2005) Ab initio calculations of strongly correlated electrons; antiferromagnetic ground state of UO2. Nucl Eng Technol 37:293–298
Mayernick AD, Janik MJ (2008) Methane activation and oxygen vacancy formation over CeO2 and Zr, Pd substituted CeO2 surfaces. J Phys Chem C 112:14955–14964
Idriss H, Al-Shankiti I, Choi YM, Al-Otaibi F (2013) Catalyst for thermochemical generation of hydrogen from water and/or thermochemical generation of carbon monoxide from carbon dioxide comprises solid solution of cerium dioxide and uranium dioxide. Patent Number(s): EP2690060-A1; WO2014016790-A1
Hinatsu Y, Fujino T (1988) Studies on magnetic properties of UO2-CeO2 solid solutions. I. Magnetic susceptibilities of solid solutions with low cerium concentrations. J Solid State Chem 73:348–355
Acknowledgments
KAUST Supercomputing Laboratory is gratefully acknowledged for the allocated computing time. Special thanks to Dr. YongMan Choi for his help in the initial part of the work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Scaranto, J., Idriss, H. The Effect of Uranium Cations on the Redox Properties of CeO2 Within the Context of Hydrogen Production from Water. Top Catal 58, 143–148 (2015). https://doi.org/10.1007/s11244-014-0353-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-014-0353-x