Abstract
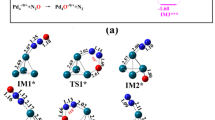
Using OpenMX quantum chemistry software for self-consistent field calculations of electronic structure with geometry optimization and 3D-RISM-KH molecular theory of solvation for 3D site distribution functions and solvation free energy, we modeled the reduction of CO2+H2 in ambient aqueous electrolyte solution of 1.0-M KH2PO4 into (i) formic acid HCOOH and (ii) CO H2O on the surfaces of Cu-, Fe-, Cu2O-, and Fe3O4-based nanocatalysts. It is applicable to its further reduction to hydrocarbons. The optimized geometries and free energies were obtained for the pathways of adsorption of the reactants from the solution, successive reduction on the surfaces of the nanocatalysts, and then release back to the solution bulk.














Similar content being viewed by others
References
Ross MB, De Luna P, Li Y, Dinh C-T, Kim D, Yang P, Sargent EH (2019). Nat Catal 2:648–658
He J, Johnson NJJ, Huang A, Berlinguette CP (2018). ChemSusChem 11:48–57
Zhou H, Liu K, Li H, Cao M, Fu J, Gao X, Hu J, Li W, Pan H, Zhan J, Li Q, Qiu X, Liu M (2019). J Colloid Interf Sci 550:17–47
Rahbari A, Ramdin M, van den Broeke LJP, Vlugt TJH (2018). Ind Eng Chem Res 57:10663–10674
Yoshio H, Katsuhei K, Shin S (1985). Chem Lett 11:1695–1698
Jitaru M, Lowy DA, Toma M, Toma BC, Oniciu LJ (1997). Appl Electrochem 27:875–889
Hori Y, Murata A, Takahashi R. J Chem Soc Farad Trans 1989, 85(8):2309–2326
Hori Y, Takahashi R, Yoshinami Y, Murata A (1997). J Phys Chem B 101:7075–7081
Kortlever R, Shen J, Schouten KJ, Calle-Vallejo F, Koper MT (2015). J Phys Chem Lett 6(20):4073–4082
Li C, Kanan MW (2012). J Am Chem Soc 134:7231–7234
Li C, Ciston J, Kanak MW (2014). Nature 508:504–507
Roberts FS, Kuhl KP, Nilsson A (2015). Angew Chem Int Ed 54:5179–5182
Kumar N, Seriani N, Gebauer R (2020 (in press)). Phys Chem Chem Phys. https://doi.org/10.1039/C9CP06453B
Wang YD, Hua XN, Zhao CC, Fu TT, Li W, Wang W (2017). Int J Hydrog Energy 42(9):5667–5675
Feaster JT, Shi C, Cave ER, Hatsukade T, Abram DN, Kuhl KP, Hahn C, Nørskov JK, Jaramillo TF (2017). ACS Catal 7:4822–4827
Choi S, Sang B-I, Hong J, Yoon KJ, Son J-W, Lee J-H, Kim B-K, Kim H (2017). Sci Rep 7:41207–41210
Kuhl KP, Hatsukade T, Cave ER, Abram DN, Kibsgaard J, Jaramillo TFJ (2014). Am Chem Soc 136:14107–14113
Kuhl KP, Cave ER, Abram DN, Jaramillo TF (2012). Energy Environ Sci 5:7050–7059
Hatsukade T, Kuhl KP, Cave ER, Abram DN, Jaramillo TF (2014). Phys Chem Chem Phys 16:13814–13819
Lejaeghere K et al (2016). Science 351((6280)):aad3000
Kovalenko A (2013). Pure Appl Chem 85:159–199
Kovalenko A (2017) Multiscale modeling of solvation. In: Breitkopf C, Swider-Lyons K (eds) Springer Handbook of Electrochemistry. Springer-Verlag, Berlin, pp 95–139 1016 p
Kovalenko A, Gusarov S (2018). Phys Chem Chem Phys 20:2947–2969
Rappe AK, Casewit CJ, Colwell KS, Goddard III WA, Skiff WMJ (1992). Am Chem Soc 114:10024–10035
Case DA, Belfon K, Ben-Shalom IY, Brozell SR, Cerutti DS, Cheatham III TE, Cruzeiro VWD, Darden TA, Duke RE, Giambasu G, Gilson MK, Gohlke H, Goetz AW, Harris R, Izadi S, Kasava-jhala K, Kovalenko A, Krasny R, Kurtzman T, Lee TS, LeGrand S, Li P, Lin C, Liu J, Luchko T, Luo R, Man V, Merz KM, Miao Y, Mikhailovskii O, Monard G, Nguyen H, Onufriev A, Pan F, Pantano S, Qi R, Roe DR, Roitberg A, Sagui C, Schott-Verdugo S, Shen J, Simmerling CL, Skrynnikov N, Smith J, Swails J, Walker RC, Wang J, Wilson L, Wolf RM, Wu X, York DM, Kollman PA (2020) AMBER 2020. University of California, San Francisco. https://ambermd.org/
Perdew JP, Burke K, Ernzerhof M (1996). Phys Rev Lett 77:3865–3868
Chandler D, McCoy JD, Singer SJ (1986) Density functional theory of nonuniform polyatomic systems. I. General Formulation. J Chem Phys 85:5971–5976
Chandler D, McCoy JD, Singer SJ (1986) Density functional theory of nonuniform polyatomic systems. II. Rational Closures for Integral Equations. J Chem Phys 85:5977–5982
Hansen J-P, McDonald IR (2006) Theory of simple liquids3rd edn. Elsevier Academic Press, London Burlington
Liu S, Tao H, Zeng L, Liu Q, Xu Z, Liu Q, Luo J-LJ (2017). Am Chem Soc 139:2160–2163
Acknowledgments
Generous computing time provided by Compute Canada/Calcul Canada (www.computecanada.ca) is acknowledged.
Funding
This work was financially supported by the National Research Council of Canada, Research Grant A1-015524-01 0002.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kovalenko, A., Neburchilov, V. Density functional theory and 3D-RISM-KH molecular theory of solvation studies of CO2 reduction on Cu-, Cu2O-, Fe-, and Fe3O4-based nanocatalysts. J Mol Model 26, 267 (2020). https://doi.org/10.1007/s00894-020-04529-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04529-8