Abstract
1,6-hexanediol (1) is an important polymer precursor for the polyester industry. In this paper, exploratory catalyst screening studies on the synthesis of 1 from 1,2,6-hexanetriol (2) are described via two different routes. The latter is available by a two-step procedure from 5-hydroxymethylfurfural (HMF, 3), a promising bio-based platform chemical. In the first approach, the direct catalytic hydrodeoxygenation of 2 to 1 with heterogeneous catalysts and molecular hydrogen was explored. Best results were obtained using a Rh–ReOx/SiO2 catalyst in water (180 °C, 80 bar H2, 20 h reaction time), leading to full conversion of 2 and 73 % selectivity to 1, the main byproduct being 1,5-hexanediol (4). In a second approach, 2 was first converted to tetrahydropyran-2-methanol (2-THPM, 5) in quantitative yield using triflic acid as catalyst (125 °C, 30 min). Various catalysts were explored for the subsequent ring opening/hydrodeoxygenation of 5 to 1 using a hydrogenation protocol and the best results were obtained with a Rh–ReOx/SiO2 catalyst, viz. 96 % selectivity to 1 at 26 % conversion (120 °C, 80 bar H2, 20 h).
Similar content being viewed by others
1 Introduction
1,6-hexanediol (1) is an important chemical for use in the production of high performance polyesters, polyurethane resins, and adhesives [1]. In 2000, the worldwide production volume of 1 was about 33,000 ton/year [2]. The major route to 1 involves the hydrogenation of adipic acid or its esters (e.g. dimethyl adipate) using heterogeneous catalysts based on carbon supported Ru–Pt–Sn [3–5], Cu–Cr–Ba oxide [6], Sn-modified Raney Ru [7], various copper-based catalysts [8, 9], CuO/ZnO/Al2O3 [10–14], and RuSn/Al2O3 [15, 16] (Eq. 1).

Our interest in this field concerns the synthesis of green 1,6-hexanediol from renewable resources. We have recently shown the proof of principle for the reaction of hydroxymethylfurfural HMF (3) to 1 via THF-dimethanol (THFDM) and 1,2,6-hexanetriol (2) as the intermediates (Eq. 2) [17].

HMF is considered to be a promising bio-based platform chemical and is regarded as a “sleeping giant” in the field of intermediate chemicals from renewable resources [18]. It is accessible by acid-catalyzed dehydration of the C6-sugars (e.g. d-glucose, d-fructose, and d-mannose) present in lignocellulosic biomass. HMF derivatives like dimethylfuran [19–21] and its ethers such as methoxymethylfurfural and ethoxymethylfurfural [22] have potential as fuel (additives), whereas 2,5-furandicarboxylic acid (FDCA) [23–31], and tetrahydrofuran-dimethanol (THFDM) may serve as building blocks in advanced polymers [32–38].
Here, we report catalytic screening studies on the synthesis of the diol 1 from triol 2. The reaction involves the selective removal of a secondary alcohol in the presence of two primary alcohols. A well-known approach involves catalytic hydrodeoxygenation using heterogeneous catalysts and, in most cases, molecular hydrogen. Examples for structurally related alcohols like 1,2-propanediol (6), 1,2-butanediol (7), 1,2-pentanediol (8), and 1,2-hexanediol (9) are provided in Table 1.
Bimetallic catalysts based on Rh–Re, Ir–Re, and Pt–W have shown to be very promising catalyst and selectivities between 73 and 90 % are reported. In some cases soluble acids (e.g. sulfuric acid) were added to improve catalytic conversions. There are also reports [46–52] on the use of homogeneous ruthenium catalysts in combination with triflic acid (HOTf) and sulfolane as the solvent for the selective hydrodeoxygenation of a secondary alcohol in the presence of a primary alcohol. For example, Schlaf et al. [51] reported the production of 1-propanol from 6 using [{Cp*Ru(CO)2}2(μ-H)] + OTf− (52 bar H2, 110 °C, 30 h) and obtained 92 % conversion with 54 % selectivity to 1-propanol. Better results were obtained [46] using [cis-Ru(6,6′-Cl2-bipy)2(OH2)2](CF3SO3)2 (48 bar H2, 125 °C, 48 h), giving 63 % yield of 1-propanol.
While preparing this manuscript, Dumesic et al. [44] reported hydrodeoxygenation reactions of various diols and triols using bimetallic Rh–ReOx catalysts on carbon supports. When using 2 as the substrate, 1 was obtained in 99.9 % at 8 % conversion (120 °C, 34 bar, 4 h). Prolonged reaction times (14 h) resulted in improved conversions (59 %), though, the selectivity to 1 was reduced considerably (62 %).
In this paper, a catalyst screening study on the conversion of 1,2,6-hexanetriol to 1,6 hexanediol using a hydrodeoxygenation approach is reported. Emphasis is on the use of bimetallic Rh–Re complexes on various supports, though monometallic catalysts have been tested as well, and the results will be compared.
2 Materials and Methods
2.1 Materials
1 (97 %) and tetrahydropyran-2-methanol 5 (98 %) were purchased from Aldrich. 2 (>97 %) was purchased from Acros. 1-propanol (>99 %) was purchased from Merck Chemicals. Sulfolane (99 %) was purchased from Aldrich. Triflic acid (99%), para-toluenesulfonic acid monohydrate (>98.5 %), aluminum chloride hexahydrate (>99 %), copper triflate (98 %), and potassium carbonate (99 %) were purchased from Aldrich.
Copper chromite catalysts were kindly supplied by BASF (Cu-1985P) and Süd-Chemie (T-4419), as well as purchased from Aldrich (product number: 209325). Copper zinc PRICAT CZ/A P and PRICAT CZ/B P catalysts were kindly supplied by Johnson Matthey. Copper zinc T-2130 was kindly supplied by Süd-Chemie. Ru/C (5 % w/w), Ru/Al2O3 (5 % w/w), Pt/C (5 % w/w), Rh/C (5 % w/w), Rh/Al2O3 (5 % w/w), Pd/C (5 % w/w), and Pd/Al2O3 (5 % w/w) were purchased from Aldrich. RuCl3 was purchased from Strem. Ru/TiO2 (5 % w/w) was purchased from Degussa and a supported nickel catalyst G-69B was kindly supplied by Süd-Chemie. RhCl3.nH2O (Rh 38–40 %), ammonium perrhenate (>99 %), ammonium molybdate tetrahydrate (MoO3 81–83 %) were purchased from Aldrich while tinchloride dihydrate (>98 %) was purchased from Riedel-de Haën. Ammonium tungsten oxide (>99.99 %) was purchased from Alfa Aesar. CARiACT G-6 3 micron silica was donated by Fuji Silysia. TiO2 (product number: 14021), SiO2–Al2O3 (grade 135), and activated carbon (product number: 484164) were purchased from Aldrich. γ-Al2O3 (product number: 044658) was purchased from Alfa Aesar. Hydrogen gas (>99.9999 %) was purchased from Hoek Loos.
2.2 Methods
2.2.1 General procedure for the preparation of the bimetallic Rh–ReOx catalysts
All catalyst preparations were carried out in air. An aqueous solution of RhCl3.nH2O(176 mg, 0.8 mmol) in water (5 mL) was added to silica (2 g, Fuji CARiACT G-6 3 micron; BET surface area 529 m2g−1 and pore volume 0.617 cm3g−1) and stirred for 2 h at room temperature. After drying at 383 K for 13–14 h, the solid was added to an aqueous solution of ammonium perrhenate (113 mg, 0.4 mmol) in water (5 mL) and stirred for 2 h, followed by drying at 383 K for 13–14 h. Calcination in air at 773 K for 3 h gave the catalyst containing 4 wt% of Rh and a Re/Rh molar ratio of 0.5.
The same procedure was used for the preparation of other Rh-based bimetallic catalysts, except for Rh–ReOx/C, where the calcination step was omitted. Ammonium molybdate tetrahydrate (131 mg, 0.1 mmol), ammonium tungsten oxide (30 mg, 0.1 mmol), and tinchloride dihydrate (94 mg, 0.4 mmol) were used for preparing the Rh–MoOx/SiO2, Rh–WOx/SiO2, and Rh–SnOx/SiO2 catalysts.
2.2.2 Reaction Procedure for the Catalyst Screening Study of 2 to 1 in 1-propanol
2 (100 mg, 0.75 mmol), catalyst (10 mg), 1-propanol (2 mL) and a Teflon stirring bar were added to an 8 mL glass vial capped with a septum, which was punctured with a short needle. The vial was placed in a stainless-steel autoclave, the autoclave was closed and stirring was started at 1,000 rpm. After three times pressurizing with first nitrogen and then hydrogen, the autoclave was pressurized with hydrogen to 10 bar and the temperature was raised to 180 °C. After 1 h, the pressure was raised to 80 bar and the reaction was continued for 3 h. Then, the autoclave was allowed to cool to ambient temperature and the pressure was released. The reactor content was filtered to remove the catalyst and the filtrate was subjected to GC analysis.
2.2.3 Reaction of 2 to 1 Using CuCr Catalysts at an Elevated Temperature
2 (500 mg, 4 mmol) dissolved in 1-propanol (30 mL) and a CuCr catalyst (100 mg) were added to a 100 mL stainless steel autoclave (Parr). The reactor was flushed three times with nitrogen and subsequently with hydrogen. After flushing, the reactor was pressurized to 100 bar, and the reaction mixture was stirred (1,000 rpm) and heated to 260 °C for 6 h. Then, the autoclave was allowed to cool to ambient temperature and the pressure was released. Product mixtures were filtered to remove the catalyst and the filtrate was subjected to GC analysis.
2.2.4 General Reaction Procedure for the Reaction of 2 to 1 in Water
2 (100 mg, 0.75 mmol), the Rh–ReOx/SiO2 catalyst (10 mg), water (2 mL) and a Teflon stirring bar were added to an 8 mL glass vial capped with a septum, which was pierced by a short needle. The vial was placed in a stainless-steel autoclave, the autoclave was closed and stirring was started at 1,000 rpm. After three times pressurizing with first nitrogen and then hydrogen, the autoclave was pressurized with hydrogen to 10 bar and the temperature was raised to 180 °C. After 1 h, the pressure was raised to 80 bar and the reaction was continued for 3 h. Then, the autoclave was allowed to cool to ambient temperature and the pressure was released. The reactor content was filtered to remove the catalyst and the filtrate was subjected to GC analysis.
2.2.5 Cyclization of 2 to 5
In a 100 mL three-neck round bottom flask, 2 (3.354 g, 25 mmol) was dissolved in sulfolane (25 mL). Then, triflic acid (13.3 μL, 0.15 mmol) was added and the reaction mixture was heated to 125 °C for 30 min. The reaction mixture was cooled and analysed using GC-FID, GC–MS and 1H- and 13C-NMR.
2.2.6 General Procedure for the Reaction of 5 to 1 in Water
The procedure described here is for the Rh–ReOx/SiO2 catalyst. The same procedure was used for all other catalysts. 5 (100 mg, 0.9 mmol), the Rh–ReOx/SiO2 catalyst (10 mg), water (2 mL) and a Teflon stirring bar were added to a glass vial and the hydrogenation was performed as described above for the hydrogenolysis of 2, except that the content was stirred for 3.5 h at 80 bar instead of 3 h. Product mixtures were filtered to remove the catalyst and the filtrate was subjected to GC analysis.
2.2.7 Product Analyses
Gas chromatography using a CP-WAX57CB column (25 m length, 0.2 mm internal diameter, and 0.25 μm film thickness) and a flame ionization detector (GC-FID) was used for product identification and quantification. The injector and the detector temperature were set at 250 °C. The oven temperature was kept at 40 °C for 5 min then heated up to 180 °C with a heating rate of 5 °C/min and to 230 °C with a heating rate of 10 °C/min and kept at this temperature for 15 min. A split ratio of 50 was used. Helium was used as the carrier gas with a flow rate of 1.1 mL/min. Toluene was used as an internal standard for the GC analysis.
GC-MS analyses was performed on a Hewlett-Packard 5890 gas chromatograph equipped with a quadrupole Hewlett-Packard 6890 MSD selective detector and a 30-m × 0.25-mm internal diameter ×0.25-μm-film sol–gel capillary column. The injector temperature was set at 250 °C. The oven temperature was kept at 40 °C for 5 min, then increased to 250 °C at a heating rate of 3 °C/min, and then held at 250 °C for 10 min.
3 Results and Discussion
3.1 One-step Conversion of 2 to 1
3.1.1 Exploratory Catalyst Screening Studies
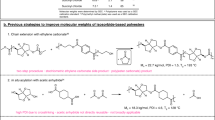
A wide range of catalysts were screened for the catalytic hydrodeoxygenation of 2 to 1 in 1-propanol as the solvent (180 °C, 3 h, 80 bar) including CuCr (three types), CuZn (three types), Ru-based catalysts (Ru/C, Ru/TiO2, Ru/Al2O3), Rh-based catalysts (Rh–ReOx/SiO2, Rh/C, Rh/Al2O3, Rh/SiO2), Pd-based catalysts (Pd/Al2O3, Pd/C), Pt/C, and Ni/kieselguhr (promoted with zirconium). Most of the catalysts were not active at the prevailing reaction condition, the only exceptions being Ru/C and the bimetallic Rh–ReOx on silica. Activity though, was very low and the conversion of 2 was less than 10 % (5 % for Rh–ReOx/SiO2 and 8 % for Ru/C) with a selectivity to 1 of 57 % for Rh–ReOx/SiO2 and 56 % for Ru/C. The byproduct for the reaction with Rh–ReOx/SiO2 catalyst was the undesired 1,5-hexanediol 4 (Eq. 3), byproducts for the reaction with Ru/C were not identified. It is clear that both Ru/C and Rh–ReOx/SiO2 showed similar performance.

The screening studies revealed that CuCr catalysts were not active under the prevailing reaction conditions. This is remarkable as Utne and co-workers reported the use of copper chromite catalyst for the direct conversion of THFDM to 1, most likely also involving 2 as an intermediate. However, the reaction conditions used by Utne (300 °C and 380 bar) to obtain a 40–50 % yield of 1 were far more severe than used here [53]. Thus, additional experiments were performed with CuCr (BASF) at elevated temperatures and pressures (260 °C, 100 bar, 6 h, 1-propanol) using 2 as the substrate. At these conditions, the catalyst is indeed active and 93 % conversion of 2 was achieved. The selectivity to 1, though, was relatively low (46 %), giving a yield of 43 %, in the same range as observed by Utne under more severe conditions. Byproducts were the diol 4 (25 %), tetrahydropyran-2-methanol (5, 11 %), 1-hexanol (10, 10 %), and 1,5-pentanediol (11, 8 %), (Eq. 4).

3.1.2 Detailed Studies Using Bimetallic Rh–Re Catalysts
Further investigations aimed to increase the yield of 1,6-hexanediol were performed using bimetallic Rh–Re catalysts on various supports in water as solvent (Table 2).
The conversion and selectivity to 1 were considerably higher in water than in 1-propanol, the solvent in the screening study. These differences in catalytic performances may be due to competitive adsorption of the solvent (1-propanol) and the substrate on the ReOx clusters. Tomishige et al. [54] proposed a mechanism for the reaction of THF-alcohol to 1,5 pentanediol using Rh–Re catalysts. It involves coordination of the OH group of the substrate to Re and subsequent hydrogenolysis of the C–O bond by the neighboring Rh centre. Thus, it is possible that water is bound more weakly to Re than 1-propanol, leading to enhanced reaction rates.
The possible positive effects of enhanced acidity on catalyst performance, as observed in the literature (Table 1), was probed by investigating the use of more acidic supports, either by using bimetallic Rh–Re catalyst on γ-alumina and mixed silica-alumina supports or the addition of γ-alumina to a bimetallic Rh–Re catalyst on silica. The use of a Rh–Re catalyst on alumina for alcohol deoxygenation studies has to the best of our knowledge not been reported before.
Reaction using Rh–Re on silica in combination with alumina (without Rh–Re), led to slightly higher conversions than for the reaction in the absence of alumina (22 vs. 17 %), though the selectivity to 1 was slightly reduced (69 % vs. 73 %). Thus, it seems that catalyst activity is positively affected by the addition of alumina.
Reactions at standard conditions with a Rh–Re catalyst on a mixed silica-alumina support led to considerably lower conversions compared to the silica only catalyst (7 vs. 17 %). Further prolongation of the reaction time to 20 h led to 20 % conversion with 76 % selectivity to 1. The selectivity at this conversion level is similar to that of the silica only catalyst. Thus, the use of mixed alumina-silica supports leads to a considerable reduction in catalyst activity, though the selectivity is comparable with that of silica at similar conversion levels. Apparently, silica is essential for high catalyst activity.
The use of a bimetallic Rh–Re catalyst on γ-alumina led to low catalyst activities (3 % conversion vs. 17 % for silica), in line with the results for the mixed silica-alumina catalyst. Thus, the use of alumina instead of silica or partial substitution of silica by alumina has a negative effect on catalyst performance.
Experiments with the standard Rh–Re catalyst on silica in the presence of K2CO3 gave a negligible conversion at standard conditions, an indication that bases have a negative effect on the reaction rates. Similar observations were reported by Dumesic and co-workers [44] for the conversion of 5 using Rh–ReOx catalysts on carbon in the presence of 0.1 M NaOH (120 °C, 34 bar H2, 4 h).
The effects of the process conditions (temperature, reaction time) on the hydrogenation of 2 were investigated using the Rh–Re on silica catalyst (Table 3). Temperature has a profound effect on catalyst activity and conversions of 2 increased from 9 % to near quantitative conversion at 180 °C for reaction times of 20–24 h. The almost constant selectivity to 1 at the different conversion levels (67–73 %) is remarkable. It suggests that the activation energy for the desired reaction to 1 is rather similar to that of the undesired reaction to 4.
3.2 Two-step Synthetic Approach Via Tetrahydropyran-2-methanol
Experiments on the conversion of 2 using CuCr catalysts in 1-propanol at elevated pressures and temperatures (vide supra) resulted in the formation of tetrahydropyran-2-methanol 5 as a side product (Eq. 4). This compound may be considered as an intermediate in the reaction sequence, as it is formed by an intramolecular etherification of 2, and a ring opening reaction could either lead to diol 1 or 9 (Eq. 5). This observation triggered us to perform additional catalytic hydrogenation experiments using 5 as the starting material.

Synthetic methodology for the synthesis of 5 has been reported; examples are the oxidative cyclization reaction of 5-hexen-1-ol [55–57] using TS-1 (60 °C, 6 h) [55, 56] or a PNIPAAm-PW12O40 3− complex (60 °C, 6 h) [57] to give 90 % and 70 % yield of 5, respectively. The synthesis of 5 from 2 [58] using BuSnCl3 as the catalyst (230 °C, 3 h) has also been explored and 5 was obtained in 60 % yield.
An improved synthetic procedure was developed by us involving the acid catalysed ring-closure of 2 using triflic acid in sulfolane at 125 °C. After 30 min, conversion was quantitative and 5 was the sole product (GC and GC–MS), indicating that it is a very viable alternative for the synthetic methodology using n-butyltin trichloride.
A wide range of catalysts was screened for the catalytic hydrogenolysis reaction of pyran 5 to 1 using hydrogen gas (180 °C, 80 bar, 3.5 h, water as solvent). Ru-based catalysts (Ru/C, Ru/Al2O3), Pd-based catalysts (Pd/C, Pd/Al2O3), Cu-based catalysts (CuCr, CuZn), Rh-based catalysts (Rh/C, Rh/SiO2, Rh/Al2O3), Pt/C, and Ni/kieselguhr (promoted with zirconium) were not active at the prevailing reaction conditions. More promising results were obtained with bimetallic Rh-based catalysts on various supports (Table 4).
Four catalysts (Rh–ReOx/SiO2, Rh–ReOx/TiO2, Rh–ReOx/SiO2-Al2O3, Rh–MoOx/SiO2) gave up to 5–12 % conversion with 100 % selectivity to 1. The activity is a function of the support type and best results were obtained with titania. Surprisingly, the Rh–ReOx on carbon catalyst is inactive, an observation not in line with literature data [44, 45]. Tomishige [45] reported 36 % conversion with 97 % selectivity to 1 (100 °C, 80 bar, 24 h) whilst Dumesic [44] obtained 27 % conversion with 97 % selectivity to 1 (120 °C, 34 bar, 4 h) using carbon supported catalysts. A possible explanation for these differences in catalytic performance is the use of a different catalyst preparation protocol. We did not perform a calcination step after catalyst synthesis to avoid partial destruction of the C support.
Replacement of Re in the bimetallic Rh–Re catalysts with other metals only led to an active catalyst in case of Mo. W and Sn promoted Rh-catalysts were not active.
Further improvements in catalytic performance were explored by variation of the Rh content of the catalyst at a fixed Rh–Re ratio and the results are provided in Table 5. Higher Rh contents (6.5 wt%) led to higher conversions but the selectivity to 1 decreased from 100 to 71 %. Also a reaction using this catalyst was performed with a higher catalyst intake (20 wt%) on substrate. Essential quantitative conversion was obtained (96 %), however the selectivity to 1 was only 55 %, the main byproduct being 1,2-hexanediol (9).
The effect of reaction conditions and particularly reaction time and temperature on catalyst performance of the Rh–Re/silica catalyst was determined (Table 6). A low conversion, though with 100 % selectivity to 1 was obtained at 120 °C. Prolonged reaction times (20 h) at this temperature gave 26 % conversion with 96 % selectivity to 1. At elevated temperatures (180 °C) and 20 h reaction time, 86 % conversion was obtained, though the selectivity to 1 dropped to 46 %. Thus, the most promising result was obtained at 120 °C and 20 h reaction time leading to a high selectivity to 1 (96 %) at a reasonable conversion (26 %).
4 Conclusions
The catalytic synthesis of 1,6-hexanediol from 1,2,6-hexanetriol using a hydrodeoxygenation approach with heterogeneous catalysts has been explored. Various catalysts have been tested and Rh–ReOx/SiO2 catalysts were found to be the best. Two approaches were explored, a one pot approach and a two-step approach using tetrahydropyran-2-methanol 5 as the intermediate, see Eq. 6 for details.

The one-step approach gave a very promising maximum yield of 1 of 73 % at full conversion of 2. The first step in the two step approach was achieved in essentially quantitative yields. For the second step, the ring opening of 5 to diol 1, the Rh–Re catalyst showed excellent selectivity (96 %), though at a relatively low conversion level (26 %). Thus when aiming for an overall high selectivity, as preferred for bulk-chemical processes with elaborate recycle streams, the two step approach seems preferred.
References:
Figueiredo FCA, Jordão E, Carvalho WA (2008) Appl Catal A 351:259
Weissermel K, Arpe HJ (2003) Components for polyamides. In: Industrial Organic Chemistry, Wiley-VCH, Weinheim
Hara Y, Endo K (1998) Jpn. Kokai Tokkyo Koho 10306047
Kayo A, Nakamura H (2000) Jpn. Kokai Tokkyo Koho 2000327606
Hara Y, Endo K, Takahashi H (2001). Jpn. Kokai Tokkyo Koho 2001009277
Guyer A, Bieler A, Sommaruga M (1955) Helv Chim Acta 38:976
Ishimura Y, Hirayama H, Nozawa T, Monzen H (1997) Jpn. Kokai Tokkyo Koho 09059188
Pinkos R, Heimann J, Polka HM, Urtel H, Windecker G (2008) WO2008012229, to BASF AG
Pinkos R, Breuninger D, Tebben GD (2010) WO2010115798, to BASF AG
Yuan P, Liu Z, Hu T, Sun H, Liu S (2010) Reac Kinet Mech Cat 100:427
Lin P et al (2005) Faming Zhuanli Shenqing Gongkai Shuomingshu 1565729
Cheng G, Shi J, Zhang Y, Huang J, Shi M, Li M (2006) Jingxi Huagong Zhongjianti 36:67
Cheng G et al (2008) Faming Zhuanli Shenqing Gongkai Shuomingshu 101113128
Cheng G et al (2008) Faming Zhuanli Shenqing Gongkai Shuomingshu 101265158
Santos SM, Silva AM, Jordão E, Fraga MA (2004) Catal Commun 5:377
Silva AM, Santos OAA, Morales MA, Baggio-Saitovitch EM, Jordão E, Fraga MA (2006) J Mol Catal A-Chem 253:62
Buntara T, Noel S, Phua PH, Cabrera IM, de Vries JG, Heeres HJ (2011) Angew Chem Int Ed 50:7083
Bicker M, Hirth J, Vogel H (2003) Green Chem 5:280
Román-Leshkov Y, Barrett CJ, Liu ZY, Dumesic JA (2007) Nature 447:982
Chidambaram M, Bell AT (2010) Green Chem 12:1253
Thananatthanachon T, Rauchfuss TB (2010) Angew Chem Int Ed 49:6616
Gruter GJM, Dautzenberg F (2007) European Patent 1834951, to Furanix Tecnologies BV
Ribeiro ML, Schuchardt U (2003) Catal Commun 4:83
Partenheimer W, Grushin VV (2001) Adv Synth Catal 343:102
Miura T, Kakinuma H, Kawano T, Matsuhisa H (2007) US20070232815, to Canon Kabushiki Kaisha
Davis SE, Houk LR, Tamargo EC, Datye AK, Davis RJ (2011) Catal Today 160:55
Pasini T, Piccinini M, Blosi M, Bonelli R, Albonetti S, Dimitratos N, Lopez-Sanches JA, Sankar M, He Q, Kiely CJ, Hutchings GJ, Cavanai F (2011) Green Chem 13:2091
Gupta NK, Nishimura S, Takagaki A, Ebitani K (2011) Green Chem 13:824
Lilga MA, Hallen RT, Gray M (2010) Top Catal 53:1264
Gorbanev YY, Klitgaard SK, Woodley JM, Christensen CH, Riisager A (2009) ChemSusChem 2:672
Casanova O, Iborra S, Corma A (2009) ChemSusChem 2:1138
Haworth WN, Jones WGM, Wiggins LF (1945) J Chem Soc 1
Utne T, Garber JD, Jones RE (1963) US Patent 3083236, to Merck & Co, Inc.
Hales RA (1962) US patent 3040062, to Atlas Chemical Industries, Inc.
Schiavo V, Descotes G, Mentech J (1991) Bull Soc Chim Fr 128:704
Lilga MA, Hallen RT, Werpy TA, White JF, Holladay JE, Frye JG Jr, Zacher AH (2007) US20070287845, to Batelle Memorial Institute
Sanborn AJ, Bloom PD (2006) US20060128843, to Archer Daniels Midland
Connolly TJ et al (2010) Org Proc Res Dev 14:459
Qin LZ, Song MJ, Chen CL (2010) Green Chem 12:1466
Furikado I, Miyazawa T, Koso S, Shimao A, Kunimori K, Tomishige K (2007) Green Chem 9:582
Amada Y, Koso S, Nakagawa Y, Tomishige K (2010) ChemSusChem 3:728
Nakagawa Y, Shinmi Y, Koso S, Tomishige K (2010) J Catal 272:191
Sato S, Takahashi R, Sodesawa T, Honda N (2004) J Mol Catal A-Chem 221:177
Chia M, Pagán-Torres YJ, Hibbitts D, Tan Q, Pham HN, Datye AK, Neurock M, Davis RJ, Dumesic JA (2011) J Am Chem Soc 133:12675
Chen K, Koso S, Kubota T, Nakagawa Y, Tomishige K (2010) ChemCatChem 2:547
Xie Z, Schlaf M (2005) J Mol Catal A-Chem 229:151
Thibault ME, DiMondo DV, Jennings M, Abdelnur PV, Eberlin MN, Schlaf M (2011) Green Chem 13:357
Ghosh P, Fagan PJ, Marshall WJ, Hauptman E, Bullock RM (2009) Inorg Chem 48:6490
Taher D, Thibault ME, DiMondo D, Jennings M, Schlaf M (2009) Chem Eur J 15:10132
Dykeman RR, Luska KL, Thibault ME, Jones MD, Schlaf M, Khanfar M, Taylor NJ, Britten JF, Harrington L (2007) J Mol Cata. A-Chem 277:233
Schlaf M, Ghosh P, Fagan PJ, Hauptman E, Bullock RM (2009) Adv Synth Catal 351:789
Schlaf M, Ghosh P, Fagan PJ, Hauptman E, Bullock RM (2001) Angew Chem Int Ed 40:3887
Utne T, Jones RE, Garber JD (1962) US Patent 3070633
Koso S, Furikado I, Shimao A, Miyazawa T, Kunimori K, Tomishige K (2009) Chem Commun 2035
Bhaumik A, Tatsumi T (1999) J Catal 182:349
Bhaumik A, Tatsumi T (1998) Chem Commun 463
Hamamoto H, Suzuki Y, Takahashi H, Ikegami S (2007) Adv Synth Catal 349:2685
Marton D, Slaviero P, Tagliavini G (1989) Tetrahedron 45:7099
Acknowledgments
We would like to thank ACTS-ASPECT for providing a grant to perform this research (ASPECT Project 053.62.017), and Rudy Parton, Rob Meier (DSM), Peter Witte, Peter Berben (BASF), Annemarie Beers (Norit), Jean Paul Lange (Shell), and Bart Zwijnenburg (Johnson Matthey) for stimulating discussions and supply of catalysts.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Buntara, T., Noel, S., Phua, P.H. et al. From 5-Hydroxymethylfurfural (HMF) to Polymer Precursors: Catalyst Screening Studies on the Conversion of 1,2,6-hexanetriol to 1,6-hexanediol. Top Catal 55, 612–619 (2012). https://doi.org/10.1007/s11244-012-9839-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-012-9839-6