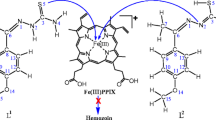
Abstract
The X-ray crystal structures of complexes between the antimalarial drugs quinine, quinidine and halofantrine and their biological target, iron(III) ferriprotoporphyrin IX (FePPIX), have been reported in the literature (de Villiers et al. in ACS Chem Biol 7:666, 2012; J Inorg Biochem 102:1660, 2008) and show that all three drugs utilize their zwitterionic alkoxide forms to coordinate to the iron atom via Fe–O bonds. In this work, density functional theory calculations with implicit solvent corrections have been used to model the energetics of formation of these complexes. It is found that the cost of formation of the active zwitterionic form of each drug is more than offset by the energy of its binding to FePPIX, such that the overall energies for complexation of all three drugs with FePPIX are moderately favourable in water, and rather more favourable in n-octanol as solvent. The calculations have been extended to develop an analogous model for the complex between FePPIX and chloroquine, whose structure is not presently known from experiment.



Similar content being viewed by others
Notes
Throughout this paper, FePPIX is used to indicate the generic form of ferriprotoporphyrin IX. Specific neutral and ionized forms of the protoporphyrin IX ligand are denoted as PPIXH2, PPIXH− and PPIX2−.
References
World Health Organization World malaria report 2013. http://www.who.int/malaria/publications/world_malaria_report_2013/en
Robert A, Coppel Y, Meunier B (2002) Chem Commun 414–415
de Villiers KA, Gildenhuys J, le Roex T (2012) ACS Chem Biol 7:666
de Villiers KA, Marques HM, Egan TJ (2008) J Inorg Biochem 102:1660
Hempelmann E (2007) Parasitol Res 100:671
Weissbuch I, Leiserowitz L (2008) Chem Rev 108:4899
Combrinck JM, Mabotha TE, Ncokazi KK, Ambele MA, Taylor D, Smith PJ, Hoppe HC, Egan TJ (2013) ACS Chem Biol 8:133
Fitch CD (2004) Life Sci 74:1957
Egan TJ (2008) J Inorg Biochem 102:1288
Pisciotta JM, Sullivan D (2008) Parasitol Int 57:89
Hayward R, Saliba KJ, Kirk K (2006) J Cell Sci 119:1016
Krafts K, Hempelmann E, Skórska-Stania A (2012) Parasitol Res 111:1
Walczak MS, Lawniczak-Jablonska K, Wolska A, Sienkiewicz A, Suárez L, Kosar AJ, Bohle DS (2011) J Phys Chem B 115:1145
Walczak MS, Lawniczak-Jablonska K, Wolska A, Sikora M, Sienkiewicz A, Suárez L, Kosar AJ, Bellemare MJ, Bohle DS (2011) J Phys Chem B 115:4419
Bohle DS, Dodd EL, Kosar AJ, Sharma L, Stephens PW, Suárez L, Tazoo D (2011) Angew Chem Int Ed 50:6151
Kuter D, Benjamin SJ, Egan TJ (2014) J Inorg Biochem 133:40
Gildenhuys J, le Roex T, Egan TJ, de Villiers KA (2013) J Am Chem Soc 135:1037
Durrant MC (2014) Dalton Trans 43:9754
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09 revision C.01. Gaussian, Wallingford
(2011) Hyperchem release 8.0.10 for windows. Hypercube
Ugliengo P, Viterbo D, Chiari G (1993) Z Kristallogr 207:9
Farrugia LJ (1997) Ortep-3 for windows, version 2.02. J Appl Crystallogr 30:565
Wong MW (1996) Chem Phys Lett 256:391. http://cccbdb.nist.gov/vibscalejust.asp
Schneider HJ, Tianjun L, Sirish M, Malinovski V (2002) Tetrahedron 58:779
Kuter D, Chibale K, Egan TJ (2011) J Inorg Biochem 105:684
de Villiers KA, Kaschula CH, Egan TJ, Marques HM (2007) J Biol Inorg Chem 12:101
Blank O, Davioud-Charvet E, Elhabiri M (2012) Antiox Redox Signal 17:544
Acknowledgments
Thanks are due to Prof. Tim Egan, University of Cape Town, and to Dr. Katherine de Villiers-Chen, Stellenbosch University, for helpful discussions, and to Northumbria University for the provision of computing facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Dr. David R. M. Walton, Founding Editor, Transition Metal Chemistry.
Rights and permissions
About this article
Cite this article
Acharige, A.M.D.S.D., Durrant, M.C. An atomic scale mechanism for the antimalarial action of chloroquine from density functional theory calculations. Transition Met Chem 39, 721–726 (2014). https://doi.org/10.1007/s11243-014-9868-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-014-9868-z