Abstract
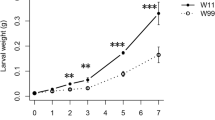
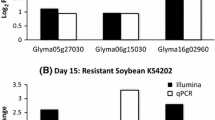
Soybean (Glycine max) is an important economic crop worldwide that has been affected by common cutworm (Spodoptera litura Fabricius) in southern China. In this study, RNA-Seq was applied to extend our understanding of the functional complexity of the soybean transcriptome in response to insects. Totals of 1004 and 1580 unigenes were differentially expressed in resistant and susceptible soybean lines, respectively. The functional classification of these unigenes identified the same significant categories as in the previous microarray results, most of which were related to stress and defense responses. A qRT-PCR analysis of 21 selected genes confirmed the results of the RNA-Seq analysis. The integration of RNA-Seq, microarray and qRT-PCR data highlighted GmVSPβ and GmN:IFR as two candidate genes that may confer insect resistance. A functional analysis of GmVSPβ and GmN:IFR revealed a certain degree of resistance to common cutworm in overexpressing tobacco lines, and GmVSPβ was more efficient in this role, which may be associated with JA signaling regulation and nicotine biosynthesis. Taken together, our studies provide an overview and survey of the genes that are involved in insect resistance. Transgenic studies of two herbivory-regulated genes demonstrated their function in insect resistance and indicated their potential application to enhance insect resistance in crops.








Similar content being viewed by others
References
Alan AR, Earle ED (2012) Plant disease resistance enhancement via transgenic expression of antimicrobial peptide MSI-99 gene in crop plants. J Biotechnol 161:14
Alfano JR, Collmer A (2004) Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annu Rev Phytopathol 42:385–414
Alignan M, Hewezi T, Petitprez M, Dechamp-Guillaume G, Gentzbittel L (2006) A cDNA microarray approach to decipher sunflower (Helianthus annuus) responses to the necrotrophic fungus Phoma macdonaldii. New Phytol 170:523–536
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Audic S, Claverie JM (1997) The significance of digital gene expression profiles. Genome Res 7:986–995
Barz W, Welle R (1992) Biosynthesis and metabolism of isoflavones and pterocarpan phytoalexins in chickpea, soybean and phytopathogenic fungi. In: Stafford HA, Ibrahim RK (eds) Phenolic metabolism in plants. Springer, New York, pp 139–164
Belles-Isles J, Dusabenyagasani M, Tremblay FM (2001) An improved procedure for production of white spruce (Picea glauca) transgenic plants using Agrobacterium tumefaciens. J Exp Bot 52:2089–2095
Bellini C, Giordani C, Lupotto E, Locatelli F, Cuzzoni E, Avogadro E, Sala F (1992) Stability of a foreign gene in transgenic Nicotiana tabacum L. plants during a cycle of dedifferentiation/differentiation. Plant Sci 82(2):193–200
Bhat SR, Srinivasan S (2002) Molecular and genetic analyses of transgenic plants: considerations and approaches. Plant Sci 163(4):673–681
Blair MW, Muñoz C, Garza R, Cardona C (2006) Molecular mapping of genes for resistance to the bean pod weevil (Apion godmani Wagner) in common bean. Theor Appl Genet 112:913–923
Broadway RM, Duffey SS (1986) Plant proteinase inhibitors: mechanism of action and effect on the growth and digestive physiology of larval Heliothis zea and Spodoptera exiqua. J Insect Physiol 32:827–833
Cassab GI (1998) Plant cell wall proteins. Annu Rev Plant Biol 49:281–309
Casu RE, Selivanova A, Perroux JM (2012) High-throughput assessment of transgene copy number in sugarcane using real-time quantitative PCR. Plant Cell Rep 31(1):167–177
Chen H, Jones AD, Howe GA (2006) Constitutive activation of the jasmonate signaling pathway enhances the production of secondary metabolites in tomato. FEBS Lett 580:2540–2546
Chen H, Seguin P, Archambault A, Constan L, Jabaji S (2009) Gene expression and isoflavone concentrations in soybean sprouts treated with chitosan. Crop Sci 49:224–236
Cheong YH, Chang HS, Gupta R, Wang X, Zhu T, Luan S (2002) Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol 129:661–677
Chung HS, Koo AJ, Gao X, Jayanty S, Thines B, Jones AD, Howe GA (2008) Regulation and function of Arabidopsis Jasmonate ZIM-domain genes in response to wounding and herbivory. Plant Physiol 146:952–964
Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21:3674–3676
Constabel CP, Bergey DR, Ryan CA (1996) Polyphenol oxidase as a component of the inducible defense response in tomato against herbivores. In: Phytochemical diversity and redundancy in ecological interactions. Springer, New York, pp 231–252
Cui ZL, Gai JY (1997) A study of leaf-feeding insect species on soybeans in Nanjing area. Soybean Sci 16:12–20
DeBoer KD, Lye JC, Aitken CD, Su AKK, Hamill JD (2009) The A622 gene in Nicotiana glauca (tree tobacco): evidence for a functional role in pyridine alkaloid synthesis. Plant Mol Biol 69:299–312
Després L, David JP, Gallet C (2007) The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol Evol 22:298–307
DeWald DB, Mason HS, Mullet JE (1992) The soybean vegetative storage proteins VSP alpha and VSP beta are acid phosphatases active on polyphosphates. J Biol Chem 267:15958–15964
Dixon RA, Achnine L, Kota P, Liu CJ, Reddy MS, Wang L (2002) The phenylpropanoid pathway and plant defence: a genomics perspective. Mol Plant Pathol 3:371–390
Du B, Zhang W, Liu B, Hu J, Wei Z, Shi Z, He R, Zhu L, Chen R, Han B, He G (2009) Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc Natl Acad Sci USA 106:22163–22168
Edens RM, Anand SC, Bolla RI (1995) Enzymes of the phenylpropanoid pathway in soybean infected with Meloidogyne incognita or Heterodera glycines. J Nematol 27:292–303
Ehlting J, Chowrira SG, Mattheus N, Aeschliman DS, Arimura G-I, Bohlmann J (2008) Comparative transcriptome analysis of Arabidopsis thaliana infested by diamond back moth (Plutella xylostella) larvae reveals signatures of stress response, secondary metabolism, and signaling. BMC Genomics 9:154
Felton GW, Donato KK, Broadway RM, Duffey SS (1992) Impact of oxidized plant phenolics on the nutritional quality of dietar protein to a noctuid herbivore, Spodoptera exigua. J Insect Physiol 38:277–285
Gatehouse AM, Gatehouse JA (1998) Identifying proteins with insecticidal activity: use of encoding genes to produce insect-resistant transgenic crops. Pestic Sci 52:165–175
Gatehouse AM, Hilder VA, Gatehouse JA (1992) Control of insect pests by plant genetic engineering. Proc R Soc Edinb Sect B Biol Sci 99:51–60
Gordon AJ, Minchin FR, Skot L, James CL (1997) Stress-induced declines in soybean N2 fixation are related to nodule sucrose synthase activity. Plant Physiol 114:937–946
Govenor HL, Schultz JC, Appel HM (1997) Impact of dietary allelochemicals on gypsy moth (Lymantria dispar) caterpillars: importance of midgut alkalinity. J Insect Physiol 43:1169–1175
Green ES, Zangerl AR, Berenbaum MR (2001) Effects of phytic acid and xanthotoxin on growth and detoxification in caterpillars. J Chem Ecol 27:1763–1773
Gulati J, Kim SG, Baldwin IT, Gaquerel E (2013) Deciphering herbivory-induced gene-to-metabolite dynamics in Nicotiana attenuata tissues using a multifactorial approach. Plant Physiol 162:1042–1059
Guo AY, Zhu QH, Chen X, Luo JC (2007) GSDS: a gene structure display server. Yichuan 29:1023–1026 [in Chinese]
Halitschke R, Baldwin IT (2003) Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. Plant J 36(6):794–807
Hart SV, Kogan M, Paxton JD (1983) Effect of soybean phytoalexins on the herbivorous insects Mexican bean beetle and soybean looper. J Chem Ecol 9:657–672
Harvey JA, van Dam NM, Witjes L, Soler R, Gols R (2007) Effects of dietary nicotine on the development of an insect herbivore, its parasitoid and secondary hyperparasitoid over four trophic levels. Ecol Entomol 32:15–23
Heim U, Weber H, Bäumlein H, Wobus U (1993) A sucrose-synthase gene of Vicia faba L.: expression pattern in developing seeds in relation to starch synthesis and metabolic regulation. Planta 191:394–401
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SA, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Howe GA (2008) New weapons and a rapid response against insect attack. Plant Physiol 146:832–838
Iseli C, Jongeneel CV, Bucher P (1999) ESTScan: a program for detecting, evaluating, and reconstructing potential coding regions in EST sequences. ISMB 99:138–148
Jouanin L, Bonadé-Bottino M, Girard C, Morrot G, Giband M (1998) Transgenic plants for insect resistance. Plant Sci 131:1–11
Kang YJ, Kim KH, Shim S, Yoon MY, Sun S, Kim MY, Van K, Lee SH (2012) Genome-wide mapping of NBS-LRR genes and their association with disease resistance in soybean. BMC Plant Biol 12:139
Kant MR, Baldwin IT (2007) The ecogenetics and ecogenomics of plant–herbivore interactions: rapid progress on a slippery road. Curr Opin Genet Dev 17:519–524
Kessler A, Halitschke R, Baldwin IT (2004) Silencing the jasmonate cascade: induced plant defenses and insect populations. Science 305:665–668
Kidd SK, Melillo AA, Lu RH, Reed DG, Kuno N, Uchida K, Furuya M, Jelesko JG (2006) The A and B loci in tobacco regulate a network of stress response genes, few of which are associated with nicotine biosynthesis. Plant Mol Biol 60:699–716
Kogan M (1972) Feeding and nutrition of insects associated with soybeans 2 Soybean resistance and host preferences of the Mexican bean beetle, Epilachna varivest. Ann Entomol Soc Am 65:675–683
Krügel T, Lim M, Gase K, Halitschke R, Baldwin IT (2002) Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology 12:177–183
Li Y, Hu Y, Bolund L, Wang J (2010) State of the art de novo assembly of human genomes from massively parallel sequencing data. Hum Genomics 4:271–277
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Mahanil S, Attajarusit J, Stout MJ, Thipyapong P (2008) Overexpression of tomato polyphenol oxidase increases resistance to common cutworm. Plant Sci 174(4):456–466
Marchetti S, Delledonne M, Fogher C, Chiaba C, Chiesa F, Savazzini F, Giordano A (2000) Soybean Kunitz, C-II and PI-IV inhibitor genes confer different levels of insect resistance to tobacco and potato transgenic plants. Theor Appl Genet 101:519–526
Mason HS, Mullet JE (1990) Expression of two soybean vegetative storage protein genes during development and in response to water deficit, wounding, and jasmonic acid. Plant Cell 2:569–579
Mishra AK, Agarwal S, Jain CK, Rani V (2009) High GC content: critical parameter for predicting stress regulated miRNAs in Arabidopsis thaliana. Bioinformation 4:151
Miyazaki K, Inoue S, Yamada K, Watanabe M, Liu Q, Watanabe T, Adachi MT, Tanaka Y, Kitajima S (2009) Differential usage of alternate promoters of the human stress response gene ATF3 in stress response and cancer cells. Nucleic Acids Res 37:1438–1451
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5:621–628
Orozco-Cardenas M, McGurl B, Ryan CA (1993) Expression of an antisense prosystemin gene in tomato plants reduces resistance toward Manduca sexta larvae. Proc Natl Acad Sci USA 90:8273–8276
Paiva NL, Edwards R, Sun Y, Hrazdina G, Dixon RA (1991) Stress responses in alfalfa (Medicago sativa L.) 11: molecular cloning and expression of alfalfa isoflavone reductase, a key enzyme of isoflavonoid phytoalexin biosynthesis. Plant Mol Biol 17:653–667
Parr JC, Thurston R (1972) Toxicity of nicotine in synthetic diets to larvae of the tobacco hornworm. Ann Entomol Soc Am 65:1185–1188
Risso D, Schwartz K, Sherlock G, Dudoit S (2011) GC-content normalization for RNA-Seq data. BMC Bioinform 12:480
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386
Russell GB, Sutherland ORW, Hutchins RFN, Christmas PE (1978) Vestitol: a phytoalexin with insect feeding-deterrent activity. J Chem Ecol 4:571–579
Sasaki-Sekimoto Y, Taki N, Obayashi T, Aono M, Matsumoto F, Sakurai N, Suzuki H, Hirai MY, Noji M, Saito K, Masuda T, Takamiya K, Shibata D, Ohta H (2005) Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J 44:653–668
Schmidt S, Baldwin IT (2009) Down-regulation of systemin after herbivory is associated with increased root allocation and competitive ability in Solanum nigrum. Oecologia 159:473–482
Schmidt GW, Delaney SK (2010) Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol Genet Genomics 283(3):233–241
Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J, Xu D, Hellsten U, May GD, Yu Y, Sakurai T, Umezawa T, Bhattacharyya MK, Sandhu D, Valliyodan B, Lindquist E, Peto M, Grant D, Shu S, Goodstein D, Barry K, Futrell-Griggs M, Abernathy B, Du J, Tian Z, Zhu L, Gill N, Joshi T, Libault M, Sethuraman A, Zhang XC, Shinozaki K, Nguyen HT, Wing RA, Cregan P, Specht J, Grimwood J, Rokhsar D, Stacey G, Shoemaker RC, Jackson SA (2010) Genome sequence of the palaeopolyploid soybean. Nature 463:178–183
Schuler TH, Poppy GM, Kerry BR, Denholm I (1998) Insect-resistant transgenic plants. Trends Biotechnol 16:168–175
Self LS, Guthrie FE, Hodgson E (1964) Adaptation of tobacco hornworms to the ingestion of nicotine. J Insect Physiol 10:907–914
Shewry PR, Lucas JA (1997) Plant proteins that confer resistance to pests and pathogens. Adv Bot Res 26:135–192
Shoji T, Ogawa T, Hashimoto T (2008) Jasmonate-induced nicotine formation in tobacco is mediated by tobacco COI1 and JAZ genes. Plant Cell Physiol 49:1003–1012
Sinha R, Kumar D, Datta R, Hazra S, Bhattacharyya D, Mazumdar AB, Mukhopadhyay R, Sultana A, Chattopadhyay S (2015) Integrated transcriptomic and proteomic analysis of Arabidopsis thaliana exposed to glutathione unravels its role in plant defense. Plant Cell Tissue Organ Cult 120:975–988
Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, Osborne E, Paredez A, Persson S, Raab T, Vorwerk S, Youngs H (2004) Toward a systems approach to understanding plant cell walls. Science 306:2206–2211
Staswick PE, Zhang Z, Clemente TE, Specht JE (2001) Efficient down-regulation of the major vegetative storage protein genes in transgenic soybean does not compromise plant productivity. Plant Physiol 127:1819–1826
Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT (2004) Nicotine’s defensive function in nature. PLoS Biol 2:e217
Sutherland OR, Russell GB, Biggs DR, Lane GA (1980) Insect feeding deterrent activity of phytoalexin isoflavonoids. Biochem Syst Ecol 8:73–75
Tiemann K, Inzé D, Montagu M, Barz W (1991) Pterocarpan phytoalexin biosynthesis in elicitor-challenged chickpea (Cicer arietinum L.) cell cultures European. J Biochem 200:751–757
Truman W, Bennett MH, Kubigsteltig I, Turnbull C, Grant M (2007) Arabidopsis systemic immunity uses conserved defense signaling pathways and is mediated by jasmonates. Proc Natl Acad Sci USA 104:1075–1080
van Dam NM, Hadwich K, Baldwin IT (2000) Induced responses in Nicotiana attenuata affect behavior and growth of the specialist herbivore Manduca sexta. Oecologia 122:371–379
Varshney RK, Nayak SN, May GD, Jackson SA (2009) Next-generation sequencing technologies and their implications for crop genetics and breeding. Trends Biotechnol 27:522–530
Vidal RO, Nascimento LCD, Maurício Costa Mondego J, Amarante Guimarães Pereira G, Falsarella Carazzolle M (2012) Identification of SNPs in RNA-seq data of two cultivars of Glycine max (soybean) differing in drought resistance. Genet Mol Biol 35:331–334
Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19:195–216
Wang H, Gao ZJ, Fan R, Zhang YS, Wu Q, Yu DY (2011) Evaluation of resistance of soybean germplasm to CCW based on three resistance mechanisms. Soybean Sci 30:8–14
Wang Y, Wang H, Fan R, Yang Q, Yu D (2014) Transcriptome analysis of soybean lines reveals transcript diversity and genes involved in the response to CCW (Spodoptera litura Fabricius) feeding. Plant Cell Environ 37:2086–2101
Wilson RF (2008) Chapter 1 Soybean: market driven research needs. In: Stacey G (ed) Genetics and genomics of soybean. Springer, New York, pp 3–15
Wu JJ, Wu Q, Wu QJ, Gai JY, Yu DY (2008) Constitutive overexpression of AOS-like gene from soybean enhanced tolerance to insect attack in transgenic tobacco. Biotechnol Lett 30:1693–1698
Yang L, Ding J, Zhang C, Jia J, Weng H, Liu W, Zhang D (2005) Estimating the copy number of transgenes in transformed rice by real-time quantitative PCR. Plant Cell Rep 23(10–11):759–763
Yauk C, Berndt M (2007) Review of the literature examining the correlation among DNA microarray technologies. Environ Mol Mutagen 48:80–394
Yuan JS, Burris J, Stewart NR, Mentewab A, Stewart CN (2007) Statistical tools for transgene copy number estimation based on real-time PCR. BMC Bioinform 8(S7):1471–2105
Zabala G, Zou J, Tuteja J, Gonzalez DO, Clough SJ, Vodkin LO (2006) Transcriptome changes in the phenylpropanoid pathway of Glycine max in response to Pseudomonas syringae infection. BMC Plant Biol 6:26
Zhang PJ, Shu JP, Fu CX, Zhou Y, Hu Y, Zalucki MP, Liu SS (2008) Trade-offs between constitutive and induced resistance in wild crucifers shown by a natural but not an artificial elicitor. Oecologia 157:83–92
Zhang J, Lu L, Ji L, Yang G, Zheng C (2009) Functional characterization of a tobacco matrix attachment region-mediated enhancement of transgene expression. Transgenic Res 18(3):377–385
Zhu-Salzman K, Ahn JE, Salzman RA, Koiwa H, Shade RE, Balfe S (2003) Fusion of a soybean cysteine protease inhibitor and a legume lectin enhances anti-insect activity synergistically. Agric Forest Entomol 5:317–323
Acknowledgments
This work was supported in part by the Key Transgenic Breeding Program of China (2013ZX08004-003), National Natural Science Foundation of China (31201230, 31171573), Jiangsu Provincial Support Program (BK2012768, BE2012328), Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT13073) and Jiangsu Collaborative Innovation Center for Modern Crop Production (JCIC-MCP).
Author contribution statement
Yongli Wang designed and performed research, analyzed data and wrote the manuscript. Hui Wang and Qing Yang participated in the improvement of the manuscript. Yujie Ma participated in the insect bioassay of transgenic tobacco lines. Wenming Yang participated in the assessment of transgene copy numbers experiment. Deyue Yu designed the research and revised the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, H., Ma, Y. et al. Identification of soybean herbivory-regulated genes and a transgenic investigation of their potential in insect resistance. Plant Cell Tiss Organ Cult 123, 321–340 (2015). https://doi.org/10.1007/s11240-015-0837-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0837-9