Abstract
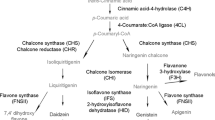
The major phytoalexin in alfalfa is the isoflavonoid (−)-medicarpin (or 6aR, 11aR)-medicarpin. Isoflavone reductase (IFR), the penultimate enzyme in medicarpin biosynthesis, is responsible for introducing one of two chiral centers in (−)-medicarpin. We have isolated a 1.18 kb alfalfa cDNA (pIFRalf1) which, when expressed in Escherichia coli, converts 2′-hydroxyformononetin stereospecifically to (3R)-vestitone, as would be predicted for IFR from alfalfa. The calculated molecular weight of the polypeptide (35400) derived from the 954 bp open reading frame compares favorably to estimated M rs determined for IFR proteins purified from other legumes. The transcript (1.4 kb) is highly induced in elicited alfalfa cell cultures. The kinetics of induction are consistent with the appearance of IFR activity, the accumulation of medicarpin, and the observed induction of other enzymes in the pathway. Low levels of IFR transcripts were found in healthy plant parts (roots and nodules) which accumulate low levels of a medicarpin glucoside. IFR appears to be encoded by a single gene in alfalfa. The cloning of IFR opens up the possibility of genetic manipulation of phytoalexin biosynthesis in alfalfa by altering isoflavonoid stereochemistry.
Similar content being viewed by others
References
Bailey, JA, Powell, PM, Arnold, GM: The temporal relationship between host cell death, phytoalexin accumulation and fungal inhibition during hypersensitive reactions of Phaseolus vulgaris to Colltotrichum lindemuthianum. Physiol Plant Pathol 17: 329–339 (1980).
Beld, M, Martin, C, Huits, H, Stuitje, AR, Gerats, AGM: Flavonoid synthesis in Petunia hybrida: partial characterization of dihydroflavonol-4-reductase genes. Plant Mol Biol 13: 491–502 (1989).
Bless, W, Barz, W: Isolation of pterocarpan synthase, the terminal enzyme of pterocarpan phytoalexin biosynthesis in cell suspension cultures of Cicer arietinum. FEBS Lett 235: 47–50 (1988).
Dalkin, K, Edwards, R, Edington, B, Dixon, RA: Stress responses in alfalfa (Medicago sativa L.) I. Induction of phenylpropanoid biosynthesis and hydrolytic enzymes in elicitor-treated cell suspension cultures. Plant Physiol 92: 440–446 (1990).
Daniel, S, Tieman, K, Wittkampf, U, Bless, W, Hinderer, W, Barz, W: Elicitor-induced metabolic changes in cell cultures of chickpea (Cicer arietinum L.) cultivars resistant and susceptible to Ascochyta rabiei. Planta 182: 270–278 (1990).
Dewick, PM: Biosynthesis of pterocarpan phytoalexins in Trifolium pratense. Phytochemistry 16: 93–97 (1977).
Dixon, RA, Fuller, KW: Effect of sunthetic auxin levels on phaseollin production and phenylalanine ammonia-lyase (PAL) activity in tissue cultures of Phaseolus vulgaris L. Physiol Plant Pathol 9: 299–312 (1976).
Dixon, RA, Harrison, MJ: Activation, structure and organization of genes involved in microbial defense in plants. Adv Genet 28: 165–234 (1990).
Edwards, R, Kessmann, H: Isoflavonoid phytoalexins and their biosynthetic enzymes. In: Bowles, DJ (ed) Molecular Plant Pathology: A Practical Approach, Oxford University Press, Cambridge, in press (1991).
Feinberg, AP, Vogelstein, B: A technique for radiolabelling DNA restriction endonuclease fragments to a high specific activity. Anal Biochem 137: 266–267 (1984).
Fischer, D, Ebenau-Jehle, C, Grisebach, H: Phytoalexin synthesis in soybean. Purification and characterization of NADPH: 2′-hydroxydaidzein oxidoreductase from elicitor-challenged soybean cell cultures. Arch Biochem Biophys 276: 390–395 (1990).
Fischer, D, Ebenau-Jehle, C, Grisebach, H: Purification and characterization of pterocarpan synthase from elicitor-challenged soybean cell cultures. Phytochemistry 29: 2879–2882 (1990).
Harrison MJ: Gene Expression During Tomato Seed Development. Ph.D. thesis, University of Manchester, Faculty of Technology, Manchester, UK (1987).
Higgins, VJ: Role of the phytoalexin medicarpin in three leaf spot diseases of alfalfa. Physiol Plant Path 2: 289–300 (1972).
Hunt, AG, MacDonald, MH: Deletion analysis of the polyadenylation signal of a pea ribulose-1,5-biphosphate carboxylase small-subunit gene. Plant Mol Biol 13: 125–138 (1989).
Ingham, JL: Phytoalexins from the leguminosae. In: JA, Bailey and JW, Mansfield (eds) Phytoalexins, pp. 21–80. John Wiley, New York (1982).
Jorrin, J, Dixon, RA: Stress responses in alfalfa (Medicago sativa L.) II. Purification, characterization and induction of phenylalanine ammonia-lyase isoforms from elicitor-treated cell suspension cultures. Plant Physiol 92: 447–455 (1989).
Kessmann, H, Choudhary, AD, Dixon, RA: Stress responses in alfalfa (Medicago sativa L.) III. Induction of medicarpin and cytochrome P450 enzyme activities in elicitor-treated cell suspension cultures and protoplasts. Plant Cell Rep 9: 38–41 (1990).
Kessmann, H, Edwards, R, Gono, PW, Dixon, RA: Stress responses in alfalfa (Medicago sativa L.) V. Constitutive and elicitor-induced accumulation of isoflavonoid conjugates in cell suspension cultures. Plant Physiol 94: 227–232 (1990).
Kurosawa, K, Ollis, WD, Redman, BT, Sutherland, IO, Alves, HM, Gottlieb, OR: Absolute configuration of isoflavans. Phytochemistry 17: 1423–1426 (1978).
Laemmli, UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 (1970).
Lamb, CJ, Merritt, TK, Butt, VS: Synthesis and removal of phenylalanine ammonia-lyase activity in illuminated discs of potato tuber parenchyme. Biochim Biophys Acta 582: 196–212 (1979).
Latunde-Dada, AO, Lucas, JA: Involvement of the phytoalexin medicarpin in the differential response of callus lines of lucerne (Medicago sativa) to infection by Verticillium albo-atrum. Physiol Plant Path 26: 31–42 (1985).
Latunde-Dada, AO, Dixon, RA, Lucas, JA: Induction of phytoalexin biosynthetic enzymes in resistant and susceptible lucerne callus lines infected with Verticillium albo-atrum. Physiol Mol Plant Path 31: 15–23 (1987).
Lawton, MA, Dixon, RA, Lamb, CJ: Elicitor modulation of L-phenylalanine ammonia-lyase in French bean cell suspension cultures. Biochim Biophys Acta 633: 162–175 (1980).
Lawton, MA, Lamb, CJ: Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol 7: 335–341 (1987).
Logemann, J, Schell, J, Willmitzer, L: Improved method for the isolation of RNA from plant tissues. Anal Biochem 163: 16–20 (1987).
Lütcke, HA, Chow, KC, Mickel, FS, Moss, KA, Kern, HF, Scheele, GA: Selection of AUG initiation codons differs in the plants and animals. EMBO J 6: 43–48 (1987).
Mierendorf, RC, Percy, C, Young, RA: Gene isolation by screening λgtII libraries with antibodies. Meth Enzymol 152b: 458–469 (1987).
Pearson, WR, Lipman, DJ: Improved tools for biological sequence comparison. Proc Natl Acad Sci USA 85: 2444–2448 (1988).
Sambrook, J, Fritsch, EF, Maniatis, T: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY (1989).
Schumacher, H-M, Gundlach, H, Fiedler, F, Zenk, MH: Elicitation of benzophenanthridine alkaloid synthesis in Eschscholtzia cell cultures. Plant Cell Rep 6: 410–413 (1987).
Stoessl, A: Biosynthesis of phytoalexins. In: Bailey, JA, Mansfield, JW (eds) Phytoalexins, pp. 133–180. John Wiley, New York, (1982).
Sun, Y, Wu, Q, Van, Etten, H, Hrazdina, G: Stereoisomerism in plant disease resistance: induction and isolation of the 7,2′-dihydroxy-4,5′-methylenedioxyisoflavone oxidoreductase, an enzyme introducing chirality during synthesis of isoflavonoid phytoalexins in pea (Pisum sativum L.). Arch Biochem Biophys 284: 167–173 (1991).
Sweigard, JA, Matthews, DE, Van, Etten, HD: Synthesis of the phytoalexin pisatin by a methyltransferase from pea. Plant Physiol 80: 277–279 (1986).
Tiemann, K, Hinderer, W, Barz, W: Isolation of NADPH: isoflavone oxidoreductase, a new enzyme of pterocarpan phytoalexin biosynthesis in cell suspension cultures of Cicer arietinum. FEBS Lett 213: 324–328 (1987).
Van, Etten, HD, Matthews, PS, Mercer, EH: (+)-Maackiain and (+)-medicarpin as phytoalexins in Sophora japonica and identification of the (−) isomers by biotransformation. Phytochemistry 22: 2291–2295 (1983).
Van, Etten, HD, Matthews, DE, Matthews, PS: Phytoalexin detoxification: Importance for pathogenicity and practical implications. Annu Rev Phytopath 27: 143–164 (1989).
Ward, EWB, Buzzell, RI: Influence of light, temperature and wounding on the expression of soybean genes for resistance to Phytophthora megasperma f. sp. glycinea. Physiol Plant Path 23: 401–409 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Paiva, N.L., Edwards, R., Sun, Y. et al. Stress responses in alfalfa (Medicago sativa L.) 11. Molecular cloning and expression of alfalfa isoflavone reductase, a key enzyme of isoflavonoid phytoalexin biosynthesis. Plant Mol Biol 17, 653–667 (1991). https://doi.org/10.1007/BF00037051
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00037051