Abstract
Recent gene knockout studies on mice have shown the role of toll-like receptor 9 (TLR9) in resolution of venous thromboembolism (VTE) through sterile inflammation. However, the role of a putative functional TLR9 polymorphism (rs5743836) in risk assessment of VTE recurrence remains unknown. The aim of our study was to investigate the TLR9 rs5743836 polymorphism in VTE patients and its association with the risk of VTE recurrence. We analyzed TLR9 rs5743836 polymorphism in Malmö thrombophilia study patients; a prospective follow-up study of 1465 VTE patients by Taqman PCR. From a total of 1465 VTE patients, those who had VTE before inclusion and those who died or had VTE recurrence during anticoagulant treatment were excluded (n = 415). Cox regression analyses were performed on the remaining 1050 VTE patients, including 126 (12.5%) patients that had recurrent VTE during follow-up period. TLR9 polymorphism was significantly associated with higher risk of VTE recurrence in female patients (HR 3.46, 95% CI 1.06–11.33) independent of acquired risk factors for VTE, family history, risk of thrombophilia and deep vein thrombosis (DVT) location. Similarly, in unprovoked VTE patients, TLR9 polymorphism was significantly associated with higher risk of VTE recurrence in female patients (HR 5.94, 95% CI 1.25–28.13) after adjusting for family history, risk of thrombophilia and DVT location. No association between TLR9 polymorphism and risk of VTE recurrence was found in male patients. Our results suggest that TLR9 rs5743836 polymorphism is an independent risk factor for VTE recurrence in female patients but not in males.
Similar content being viewed by others
Introduction
Venous thromboembolism (VTE) consists of two related conditions: deep vein thrombosis (DVT) and pulmonary embolism (PE) [1]. Primarily, it was considered to be a complication of hospitalization after major surgery. However, prior epidemiological studies have reported that about half of the patients, which had been diagnosed with VTE, were those who had never been hospitalized nor had any major illness [1, 2]. VTE is a potentially lethal disease that occurs with an incidence rate of 1–2 cases per 1000 person-years [2, 3]. About two-thirds of the patients diagnosed with symptomatic VTE had DVT and one-third had PE [2]. The mortality rate after 30 days of diagnosis with DVT was reported as 4.6%, with PE as 9.7 and 6.4% for patients diagnosed with VTE (both DVT and PE) [4]. VTE is a chronic disease and patients that have experienced one episode of VTE are always at the risk of recurrence and the risk is highest during the first 6–12 months [5]. The cumulative rate of VTE recurrence increases with time; 17.5% after 2 years, 24.96% after 5 years and rises up to 30.3% after 8 years from the first diagnosis with primary VTE [6]. Patients with unprovoked VTE (without known acquired risk factors for VTE, e.g., older age, immobilization, trauma, major surgery, female hormone therapy, pregnancy etc.) are at higher risk of VTE recurrence as compared to provoked VTE (patients with acquired risk factors) [7].
VTE patients are treated with standard treatment protocol, i.e., anticoagulant therapy for several months which protects patients from VTE recurrence, however, at the cost of severe bleeding [8, 9]. Regardless of the several identified risk factors, such as sex, D-dimers level and residual thrombosis, it is still difficult to precisely predict the probability of VTE recurrence after stopping the anticoagulation treatment [8–10]. Moreover, anticoagulants prevent the formation of new blood clots but do not remove the existing thrombi, which undergo a slow process of natural resolution by organization and vein recanalization [11].
Although a number of genes are identified as risk factors for VTE, the major portion of the heritability remains unknown. Twins and familial studies have reported that the heritability cause of VTE is up to 60%, and most of these genetic factors are still unknown [12, 13]. It is therefore clinically relevant to identify new biomarkers that allow early prediction of patients at risk of VTE recurrence in order to tailor anti-coagulant therapy.
VTE pathophysiology is now better understood following several experimental studies. It is believed that inflammation plays a major role in VTE pathophysiology [14]. Studies conducted in the 1970s using radiolabel leukocytes showed that there is an uptake of white blood cells in the venous thrombus [15, 16]. Furthermore, plasma levels of C-reactive protein (CRP), a prominent acute-phase reactant and inflammatory marker, are known to increase in DVT patients [17]. Moreover, clinically it is apparent that VTE patients manifest the cardinal signs of inflammation (heat, redness, pain and swelling). Thus, inflammation is considered to have a pivotal role in VTE formation.
Venous thrombus formation, propagation and its dissolution is a balance between two mechanisms, i.e. coagulation and innate immune mechanisms [14]. DVT resolution is an inflammatory process (known as sterile inflammation) which is similar to that of sterile wound healing. It occurs naturally through a process of tissue organization including neovascularization [14, 18]. Fibrinogen, in venous thrombus, along with its degradation products is present in abundance and these molecules stimulate the activation of the innate immune system to produce cytokines and chemokines that are involved in the sterile inflammatory process [19].
TLRs are important members of the innate immune system. Among the TLR family, TLR9 has been studied for its role in VTE resolution. Leukocytes (monocytes, neutrophils, lymphocytes and dendritic cells) express TLR9 on their membranes [20, 21]. Animal studies show that deletion of the TLR9 gene severely restricts the VTE resolution process, which suggests that TLR9 is an important player in venous thrombus resolution by modulating sterile inflammation [22, 23]. TLR9 gene has been found to be polymorphic in several diseases [24–27]. One of the putative functional polymorphism is TLR9 rs5743836 polymorphism (−1237T/C polymorphism), which is located within the promoter region of TLR9 gene and has been studied in several diseases including cardiovascular, cancers and autoimmune diseases [27–30]. Since this polymorphism (TLR9 rs5743836 polymorphism) is shown to affect the transcription of TLR9 gene, and TLR9 gene is involved in VTE resolution, it is worthwhile to investigate the role of TLR9 rs5743836 polymorphism in recurrent VTE patients and its association with the risk of VTE recurrence. To our knowledge, this is the first study in which TLR9 rs5743836 polymorphism has been studied in a well-established VTE cohort to investigate its association with the risk of VTE recurrence. Novel knowledge will therefore be obtained with the potential to influence future risk assessments and pharmacological preventive measures.
Materials and methods
Study subjects
A prospective population-based study of 1465 consecutive unselected VTE patients [Malmö thrombophilia study (MATS)] was performed at Skåne University Hospital. MATS is a well characterized cohort including VTE patients that were followed after inclusion (March 1998), until VTE recurrence or death of the patient or end of the study (December 2008) [31, 32]. For all MATS patients, VTE events prior to inclusion, location of DVT, immobilization and cast therapy, hospitalization, surgical intervention, malignancies that were diagnosed previously or at diagnosis of VTE, use of contraceptive pills, hormonal therapy, pregnancy and postpartum period (first 6 weeks after delivery), family history of VTE (history of VTE in first-degree relatives), and VTE recurrence during the follow-up period were recorded. All hospital records for VTE patients were screened by a research nurse. The rate of consensual participation in this study was 70%. The remaining patients (30%) were excluded from the study because they refused to participate or did not give consent for the blood sampling or could not complete the questionnaire for the risk factor analysis due to dementia, language problems or presence of other severe diseases.
The inclusion criteria in MATS were: diagnosis of DVT and/or PE. VTE diagnosis was objectively confirmed by phlebography, computed tomography (CT), duplex ultrasonography, lung scintigraphy or magnetic resonance imaging (MRI).
All MATS patients were treated according to the standard treatment protocol of Malmö University Hospital, i.e., all patients were initially treated with low molecular weight heparin or unfractionated heparin and then with warfarin as an oral anticoagulant. The hospital treatment protocol recommended therapy for 3–6 months for first-time VTE, with consideration of extended treatment in case of recurrent VTE. A total of 18% of patients were treated for >1 year or for life. Thrombophilia was defined as presence of the factor V Leiden (FVL) mutation (rs6025) or factor II G20210A mutation (rs1799963), or a level below the laboratory reference range of protein C [<0.7 kilo international unit (kIU/L)], free protein S (women < 0.5 kIU/L, men < 0.65 kIU/L) or antithrombin (<0.82 kIU/L) in patients without warfarin treatment.
Follow-up period (Mean ± SD, 3.9 ± 2.5) was counted in years after stopping the anticoagulant treatment until the diagnosis of VTE, death of the patient or the end of study (December 2008). This study was approved by the ethical committee of Lund University. All the participants provided written permission before their inclusion in the study according to the declaration of Helsinki.
Laboratory methods
DNA from the whole blood was extracted by using QiAmp 96 DNA Blood Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Genotyping of TLR9 rs5743836 polymorphism was performed by TaqMan® SNP Genotyping Assay according to the manufacturer’s protocol (Applied Biosystems, Life Technologies Corporation, Carlsbad, CA, USA). To summarize, a polymerase chain reaction (PCR) master mix was prepared as 2.5 µL Taqman master mix, 0.25 µL Taqman gene-specific assay (VIC and FAM probes for TLR9 rs5743836 polymorphism) and 0.25 µL deionized water. Master Mix (3 µL) was added to each well in 384 wells PCR plate followed by addition of 10 ng DNA. PCR plate was vortexed and centrifuged at 1000 rpm (revolutions per minute) for 30 s. BioRad CFX384 real-time PCR (1000 Alfred Nobel Drive Hercules, California 94547 USA), according to manufacturer’s instructions, was used for polymorphism analysis with the following temperature conditions: 95 °C for 10 min followed by 40 × (92 °C for 15 s, 60 °C for 1 min). Different alleles of TLR9 rs5743836 polymorphism were determined by using BioRad CFX manager software.
Analysis of known thrombophilic variants
FVL and factor II G20210A mutations in DNA were analyzed by TaqMan allele discrimination assays (Applied Biosystems) as described previously [33]. Analysis of free Protein S was performed by latex immunoassay with Coamatic® Protein S-Free (Chromogenix, Haemochrom Diagnostica AB, Gothenburg, Sweden) [34]. Protein C levels were analyzed by chromogenic method using the Berichrom® Protein C reagent (Siemens Healthcare Diagnostics, Upplands Väsby, Sweden) [35]. For antithrombin analysis, thrombin-based method using Berichrom Antithrombin (Siemens Healthcare Diagnostics) was used [36]. BCS-XP coagulation analyzer (Siemens Healthcare Diagnostics) was used to perform these analyses.
Statistical analysis
Statistical analyses were performed by using SPSS version 21 (IBM, Armonk, NY, USA). Dichotomous variables were compared by Chi square test or Fisher’s exact test, where appropriate and continuous variables were compared by Mann–Whitney U test. Interaction term analysis was performed to test for an interaction between TLR9 rs5743836 polymorphism and gender of the patients. Kaplan–Meier survival curves for time to recurrent VTE by TLR9 rs5743836 polymorphism genotypes were plotted and the log-rank test was used to compare recurrence-free survival between genotypes. Hardy–Weinberg equilibrium analysis was performed to observe the genotypic distribution. Univariate and multivariate Cox regression analyses were performed using Cox proportional hazards models, after adjusting for location of DVT, family history of VTE, mild and severe thrombophilia and acquired risk factors for VTE. For each group of patients, hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. Multivariate Cox regression analyses were performed as sensitivity analyses by including all VTE patients, with exception for those which had thrombotic events before inclusion. The follow-up period for sensitivity analyses was calculated from the time of inclusion and was adjusted for the duration of anticoagulation treatment.
Results
Clinical data of the patients
From a total of 1465 patients, those who had thrombotic events before inclusion (n = 154) were excluded. For the remaining patients (n = 1311), 148 (11.3%) had VTE recurrence during the follow-up period. Frequency of FVL mutation was higher in patients with recurrent VTE (40.8%) as compared to those with non-recurrent VTE (28.5%) (P = 0.002). Of the patients with recurrent VTE, 32.4% had a family history of VTE as compared to 23.5% in non-recurrent VTE (P = 0.024). Whereas, no significant difference was observed among recurrent and non-recurrent VTE patients in age, sex, deep vein thrombosis (DVT), pulmonary embolism (PE), body mass index (BMI), protein C, protein S and antithrombin deficiency (P > 0.05) Table 1.
TLR9 rs5743836 polymorphism has three genotypic forms, homozygous wild type (TT), heterozygous (TC) and homozygous mutated form (CC). In data analysis, all three genotypic forms were analyzed separately as well as by combining homozygous wild type and heterozygous form (Table 1).
In Hardy–Weinberg equilibrium analyses, genotypic distributions in TLR9 rs5743836 polymorphism did not deviate significantly (P > 0.05).
TLR9 rs5743836 polymorphism and risk of VTE recurrence
For the recurrence analyses of the patients (n = 1311), those who had recurrence or died during anticoagulant treatment were excluded (n = 261). Analyses were performed on the remaining 1050 patients including 126 (12%) recurrent VTE patients.
For TLR9 rs5743836 polymorphism, univariate and multivariate Cox regression analyses were performed with individual genotypes (TT, TC and CC) to investigate their association with the risk of VTE recurrence. In the whole population, there was no significant association between TLR9 rs5743836 polymorphism and risk of VTE recurrence (HR 1.12, CI 0.41–3.04, P = 0.827 and HR 0.99, CI 0.36–2.70, P = 0.981) on Uni- and multi-variate Cox regression analyses respectively. However, inclusion of an interaction term between TLR9 rs5743836 polymorphism and gender of the patients in the model showed a modifying effect of gender on TLR9 polymorphism (TLR9 polymorphism × gender: HR 2.49, 95% CI 1.08–5.74, P = 0.032). After stratification of data, according to gender, a significant association between TLR9 rs5743836 polymorphism and risk of VTE recurrence was found in female patients on univariate Cox regression analysis (HR 3.60 and 95% CI 1.11–11.61, P = 0.033) and on multivariate Cox regression analysis after adjusting for location of DVT, family history of VTE, mild and severe thrombophilia and acquired risk factors for VTE (HR 3.46, CI 1.06–11.33, P = 0.040). Similar results were found when we combined the T allele containing genotypes (TT and TC) and compared with homozygous mutated genotype (CC); only female patients having TLR9 rs5743836 polymorphism were at higher risk of VTE recurrence (HR 3.44, CI 1.07–11.0, P = 0.037 and HR 3.36, CI 1.04–10.88, P = 0.043 on univariate and multivariate Cox regression analyses respectively) (Table 2).
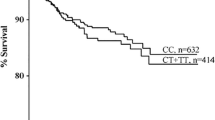
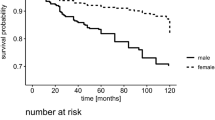
A survival analysis by Kaplan–Meier curve was performed to analyze whether TLR9 rs5743836 polymorphism influences recurrence-free survival. Patients having T and C alleles were compared and results showed a significant difference in recurrence-free survival (Fig. 1a, Log-rank test, P = 0.037) in female patients. Female patients having CC genotype had significantly shorter recurrence-free survival as compared to TT and TC genotypes, whereas no significant difference was observed between different genotypes and risk of VTE recurrence in male patients (Fig. 1b, Log-rank test, P = 0.342).
TLR9 rs5743836 polymorphism and risk of VTE recurrence in patients with unprovoked first VTE
We also performed a sub-analysis on unprovoked first VTE patients (n = 618); patients with a recorded acquired risk factor for VTE, i.e., immobilization or cast therapy within the last month, use of contraceptives pills, surgical intervention, malignancies diagnosed prior to or at diagnosis of the first VTE event, current pregnancy and postpartum period (first 6 weeks after delivery) and female hormone therapy were excluded from this analysis. Our results show an association between TLR9 rs5743836 polymorphism and risk of VTE recurrence, only in female unprovoked VTE patients, though this association did not reach statistical significance on univariate Cox regression analysis (HR 3.79, CI 0.89–16.04, P = 0.071). However, on multivariate Cox regression analysis, after adjusting with location of DVT, family history of VTE, mild and severe thrombophilia, TLR9 polymorphism was significantly associated with the risk of VTE recurrence (HR 5.94, CI 1.25–28.13, P = 0.025) (Table 3).
Furthermore, sensitivity analyses were performed including all MATS patients (n = 1311) except those that had VTE before inclusion (n = 154). The follow-up time for these analyses was calculated from the time of inclusion and was adjusted for duration of anticoagulant treatment. Multivariate Cox regression analyses (adjusted for duration of anticoagulant treatment, mild and severe thrombophilia, location of DVT, family history and acquired risk factors for VTE), showed that female patients with TLR9 rs5743836 polymorphism have significantly higher risk of VTE recurrence as compared to patients with wild-type genotype (HR 3.44, CI 1.05–11.26, P = 0.042) (Table 1 in the Supplementary Appendix).
Discussion
In the current study, we have analyzed TLR9 rs5743836 polymorphism for its role in the risk assessment of VTE recurrence. Our results show that TLR9 rs5743836 polymorphism is significantly associated with higher risk of VTE recurrence in female patients, independent of known clinical risk factors associated with VTE recurrence. The HR for VTE recurrence in female patients was 3.46. In the subgroup of unprovoked VTE recurrence in female patients, the HR was 5.94. To our knowledge, this is the first study in which TLR9 rs5743836 polymorphism has been studied in VTE recurrence. Previously, there was a single study in which this polymorphism has been studied for its role in primary deep vein thrombosis (DVT) and no association was found between TLR9 rs5743836 polymorphism and risk of DVT among a European population [27]. Recent studies have suggested that the risk factors for primary and recurrent VTE may differ [37, 38]. Furthermore, the above-mentioned study [27] included only male patients, while our results suggest that TLR9 rs5743836 polymorphism is only associated with risk of VTE recurrence in female patients but not in male patients. It is now well established that the risk of VTE recurrence varies according to the gender of the patients and risk factors for VTE recurrence are gender specific [32, 39]. Moreover, effects of sex hormones on inflammation in patients with and without TLR9 polymorphism may differ and thereby influence the risk of VTE recurrence in these patients [40, 41].
TLR9 rs5743836 polymorphism has been shown to affect the transcription activity of TLR9, CC genotype (mutant) was associated with lower transcription activity as compared to TT genotype (wild-type) [25]. Gene knockout studies have shown a pivotal role of TLR9 in VTE resolution [22, 23]. Taken together with our findings, this suggests that TLR9 polymorphism may reduce the ability of VTE resolution and therefore may increase the risk of VTE recurrence.
We further analyzed TLR9 rs5743836 polymorphism in unprovoked VTE patients, which are known to have higher risk of VTE recurrence. We found a significant association between TLR9 rs5743836 polymorphism and VTE recurrence in female patients, independent of location of DVT, family history of VTE & mild and severe thrombophilia. These results show that TLR9 rs5743836 polymorphism may also be an independent risk factor for VTE recurrence in these higher risk female patients.
TLR9 is one of the important members of pattern recognition receptors (PRRs) that are involved in thrombus resolution. Leukocytes express several pattern PRRs (including TLR9) that are activated by damaged associated molecular patterns (DAMPs) from cellular debris, causing the release of mediators that promote sterile inflammation [23]. Leukocytes, specially, neutrophils are known to be involved in the early thrombus resolution by promoting collagenolysis and fibrinolysis [42, 43] and monocytes are likely the most important cells involved in late VTE resolution [44]. Protective role of TLR9, as an integral part of thrombus resolution process, due to the rs5743836 polymorphism may be lost and therefore is associated with higher risk of VTE recurrence.
In conclusion, for the first time, we have analyzed TLR9 rs5743836 polymorphism in recurrent VTE patients. Our results suggest that TLR9 rs5743836 polymorphism is a potential marker for VTE recurrence in female but not in male patients. The findings shed new light on potential differential mechanisms by gender in the development of VTE recurrence as well as on future preventive strategies.
References
Anderson FA, Spencer FA (2003) Risk factors for venous thromboembolism. Circulation 107(23 suppl 1):I-9–I-16. doi:10.1161/01.cir.0000078469.07362.e6
White RH (2003) The epidemiology of venous thromboembolism. Circulation 107(23 suppl 1):I-4–I-8. doi:10.1161/01.cir.0000078468.11849.66
Heit JA (2008) The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 28(3):370–372. doi:10.1161/atvbaha.108.162545
Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrom J (2007) Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost 5(4):692–699. doi:10.1111/j.1538-7836.2007.02450.x
Mason C (2009) Venous thromboembolism: a chronic illness. J Cardiovasc Nurs 24(6 Suppl):S4–S7. doi:10.1097/JCN.0b013e3181b85cbb
Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E, Prins MH (1996) The long-term clinical course of acute deep venous thrombosis. Ann Intern Med 125(1):1–7
Iorio A, Kearon C, Filippucci E, Marcucci M, Macura A, Pengo V, Siragusa S, Palareti G (2010) Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med 170(19):1710–1716. doi:10.1001/archinternmed.2010.367
Kyrle PA, Eichinger S (2005) Deep vein thrombosis. The Lancet 365(9465):1163–1174. doi:10.1016/S0140-6736(05)71880-8
Carrier M, Le Gal G, Wells PS, Rodger MA (2010) Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med 152(9):578–589. doi:10.7326/0003-4819-152-9-201005040-00008
Kyrle PA, Rosendaal FR, Eichinger S (2010) Risk assessment for recurrent venous thrombosis. The Lancet 376(9757):2032–2039. doi:10.1016/S0140-6736(10)60962-2
Modarai B, Burnand KG, Humphries J, Waltham M, Smith A (2005) The role of neovascularisation in the resolution of venous thrombus. Thromb Haemost 93(5):801–809. doi:10.1160/th04-09-0596
Heit JA, Phelps MA, Ward SA, Slusser JP, Petterson TM, De Andrade M (2004) Familial segregation of venous thromboembolism. J Thromb Haemost 2(5):731–736. doi:10.1111/j.1538-7933.2004.00660.x
Larsen TB, Sorensen HT, Skytthe A, Johnsen SP, Vaupel JW, Christensen K (2003) Major genetic susceptibility for venous thromboembolism in men: a study of Danish twins. Epidemiology 14(3):328–332
Wakefield TW, Myers DD, Henke PK (2008) Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol 28(3):387–391. doi:10.1161/atvbaha.108.162289
Charkes ND, Dugan MA, Malmud LS, Stern H, Anderson H, Kozar J, 3rd, Maguire R (1974) Letter: labelled leucocytes in thrombi. Lancet 2(7880):600
Schaub RG, Simmons CA, Koets MH, Romano PJ, 2nd, Stewart GJ (1984) Early events in the formation of a venous thrombus following local trauma and stasis. Lab Invest 51(2):218–224
Bucek RA, Reiter M, Quehenberger P, Minar E (2002) C-reactive protein in the diagnosis of deep vein thrombosis. Br J Haematol 119(2):385–389
Saha P, Humphries J, Modarai B, Mattock K, Waltham M, Evans CE, Ahmad A, Patel AS, Premaratne S, Lyons OT, Smith A (2011) Leukocytes and the natural history of deep vein thrombosis: current concepts and future directions. Arterioscler Thromb Vasc Biol 31(3):506–512. doi:10.1161/atvbaha.110.213405
Szaba FM, Smiley ST (2002) Roles for thrombin and fibrin(ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood 99(3):1053–1059
Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S (2000) A Toll-like receptor recognizes bacterial DNA. Nature 408(6813):740–745. doi:10.1038/35047123
Jozsef L, Khreiss T, El Kebir D, Filep JG (2006) Activation of TLR-9 induces IL-8 secretion through peroxynitrite signaling in human neutrophils. Journal of immunology 176(2):1195–1202
Henke PK, Mitsuya M, Luke CE, Elfline MA, Baldwin JF, Deatrick KB, Diaz JA, Sood V, Upchurch GR, Wakefield TW, Hogaboam C, Kunkel SL (2011) Toll-like receptor 9 signaling is critical for early experimental deep vein thrombosis resolution. Arterioscler Thromb Vasc Biol 31(1):43–49. doi:10.1161/atvbaha.110.216317
Dewyer NA, El-Sayed OM, Luke CE, Elfline M, Kittan N, Allen R, Laser A, Oostra C, Comerota A, Hogaboam C, Kunkel SL, Henke PK (2015) Divergent effects of Tlr9 deletion in experimental late venous thrombosis resolution and vein wall injury. Thromb Haemost 114(5):1028–1037. doi:10.1160/th14-12-1031
El-Omar EM, Ng MT, Hold GL (2008) Polymorphisms in Toll-like receptor genes and risk of cancer. Oncogene 27(2):244–252. doi:10.1038/sj.onc.1210912
Novak N, Yu CF, Bussmann C, Maintz L, Peng WM, Hart J, Hagemann T, Diaz-Lacava A, Baurecht HJ, Klopp N, Wagenpfeil S, Behrendt H, Bieber T, Ring J, Illig T, Weidinger S (2007) Putative association of a TLR9 promoter polymorphism with atopic eczema. Allergy 62(7):766–772. doi:10.1111/j.1398-9995.2007.01358.x
Ng MT, Van’t Hof R, Crockett JC, Hope ME, Berry S, Thomson J, McLean MH, McColl KE, El-Omar EM, Hold GL (2010) Increase in NF-kappaB binding affinity of the variant C allele of the toll-like receptor 9—1237T/C polymorphism is associated with Helicobacter pylori-induced gastric disease. Infect Immun 78(3):1345–1352. doi:10.1128/iai.01226-09
Lazarus R, Klimecki WT, Raby BA, Vercelli D, Palmer LJ, Kwiatkowski DJ, Silverman EK, Martinez F, Weiss ST (2003) Single-nucleotide polymorphisms in the Toll-like receptor 9 gene (TLR9): frequencies, pairwise linkage disequilibrium, and haplotypes in three U.S. ethnic groups and exploratory case-control disease association studies. Genomics 81(1):85–91
dos Santos BP, Valverde JV, Rohr P, Monticielo OA, Brenol JC, Xavier RM, Chies JA (2012) TLR7/8/9 polymorphisms and their associations in systemic lupus erythematosus patients from southern Brazil. Lupus 21(3):302–309. doi:10.1177/0961203311425522
Kutikhin AG (2011) Association of polymorphisms in TLR genes and in genes of the Toll-like receptor signaling pathway with cancer risk. Hum Immunol 72(11):1095–1116. doi:10.1016/j.humimm.2011.07.307
Zhang L, Qin H, Guan X, Zhang K, Liu Z (2013) The TLR9 gene polymorphisms and the risk of cancer: evidence from a meta-analysis. PloS ONE 8(8):e71785. doi:10.1371/journal.pone.0071785
Isma N, Svensson PJ, Gottsater A, Lindblad B (2009) Prospective analysis of risk factors and distribution of venous thromboembolism in the population-based Malmo Thrombophilia Study (MATS). Thromb Res 124(6):663–666. doi:10.1016/j.thromres.2009.04.022
Ahmad A, Sundquist K, Zoller B, Dahlback B, Svensson PJ, Sundquist J, Memon AA (2016) Identification of polymorphisms in Apolipoprotein M gene and their relationship with risk of recurrent venous thromboembolism. Thromb Haemost. doi:10.1160/th16-03-0178
Sveinsdottir SV, Saemundsson Y, Isma N, Gottsater A, Svensson PJ (2012) Evaluation of recurrent venous thromboembolism in patients with Factor V Leiden mutation in heterozygous form. Thromb Res 130(3):467–471. doi:10.1016/j.thromres.2012.03.020
Giri TK, Hillarp A, Hardig Y, Zoller B, Dahlback B (1998) A new direct, fast and quantitative enzyme-linked ligandsorbent assay for measurement of free protein S antigen. Thromb Haemost 79(4):767–772
Francis RB Jr, Seyfert U (1987) Rapid amidolytic assay of protein C in whole plasma using an activator from the venom of Agkistrodon contortrix. Am J Clin Pathol 87(5):619–625
Odegard OR, Lie M, Abildgaard U (1975) Heparin cofactor activity measured with an amidolytic method. Thromb Res 6(4):287–294
van Hylckama Vlieg A, Flinterman LE, Bare LA, Cannegieter SC, Reitsma PH, Arellano AR, Tong CH, Devlin JJ, Rosendaal FR (2014) Genetic variations associated with recurrent venous thrombosis. Circ Cardiovasc Genet 7(6):806–813. doi:10.1161/circgenetics.114.000682
Zhu T, Martinez I, Emmerich J (2009) Venous thromboembolism: risk factors for recurrence. Arterioscler Thromb Vasc Biol 29(3):298–310. doi:10.1161/atvbaha.108.182428
Smith JA Jr (2005) The risk of recurrent venous thromboembolism in men and women. J Urol 173(3):886–887. doi:10.1097/00005392-200503000-00071
Fairweather D (2014) Sex differences in inflammation during atherosclerosis. Clinical medicine insights. Cardiology 8(Suppl 3):49–59. doi:10.4137/cmc.s17068
Engler H, Benson S, Wegner A, Spreitzer I, Schedlowski M, Elsenbruch S (2016) Men and women differ in inflammatory and neuroendocrine responses to endotoxin but not in the severity of sickness symptoms. Brain Behav Immun 52:18–26. doi:10.1016/j.bbi.2015.08.013
Chen GY, Nunez G (2010) Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 10(12):826–837. doi:10.1038/nri2873
Varma MR, Varga AJ, Knipp BS, Sukheepod P, Upchurch GR, Kunkel SL, Wakefield TW, Henke PK (2003) Neutropenia impairs venous thrombosis resolution in the rat. J Vasc Surg 38(5):1090–1098. doi:10.1016/s0741
Hogaboam CM, Steinhauser ML, Chensue SW, Kunkel SL (1998) Novel roles for chemokines and fibroblasts in interstitial fibrosis. Kidney Int 54(6):2152–2159. doi:10.1046/j.1523-1755.1998.00176.x
Acknowledgements
We would like to thank biobank services at Biobank, Lab medicine Skåne, Sweden. We also thank science editor Patrick Reilly for language editing of this manuscript.
Funding
This work was supported by Grants awarded to Dr Bengt Zöller by the Swedish Heart–Lung Foundation, ALF funding from Region Skåne awarded to Dr Bengt Zöller and Dr Kristina Sundquist, grants awarded to Dr Bengt Zöller and Dr Kristina Sundquist by the Swedish Research Council. The funders had no role in in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Ethical approval
This study was approved by the ethical committee of Lund University according to the declaration of Helsinki.
Informed consent
This research involving human participants and an informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ahmad, A., Sundquist, K., Zöller, B. et al. Association between TLR9 rs5743836 polymorphism and risk of recurrent venous thromboembolism. J Thromb Thrombolysis 44, 130–138 (2017). https://doi.org/10.1007/s11239-017-1491-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-017-1491-3