Abstract
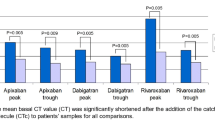
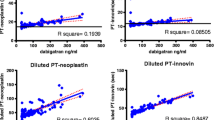
Dabigatran, a new direct thrombin inhibitor, achieves strong anticoagulation that is more predictable than warfarin. Nevertheless, a patient on dabigatran therapy (DT) may suffer from thrombotic or bleeding events. The routine monitoring of DT is not recommended, and standard coagulation tests are not sensitive enough for the assessment of DT activity. The aim of this study was to examine the clinical usefulness of the Hemoclot® Thrombin Inhibitor (HTI) assay in the assessment of dabigatran plasma levels in patients with non-valvular AF. Nineteen patients (12 men, 7 women) on DT were included in this preliminary prospective observational study. Dabigatran was administrated twice daily in a two dose regimens: 150 mg (5 patients) and 110 mg (14 patients). Blood samples were taken for the assessment of trough and peak levels of dabigatran. Dabigatran concentrations were measured with the HTI assay. The average dabigatran trough level was 69.3 ± 55.5 ng/ml and the average dabigatran peak level was 112.7 ± 66.6 ng/ml. The dabigatran trough plasma concentration was in the established reference range in 15 patients and the dabigatran peak plasma concentration was in the established reference range in 9 patients, respectively. Despite the fact that the activated partial thromboplastin and thrombin times were generally changed (prolonged), these tests failed to identify the patients with too low or too high dabigatran concentrations. The study confirmed the high sensitivity of the HTI assay for the assessment of dabigatran plasma levels. When compared to standard coagulation tests, the HTI is a more suitable assay for the monitoring of patients treated with dabigatran. Monitoring of DT may be beneficial in selected patients; however, further studies will be needed for the final clarification of this issue.
Similar content being viewed by others
References
Camm AJ, Lip GY, De Caterina R et al (2012) 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 33:2719–2747
Ahmad Y, Lip GY (2013) Warfarin for stroke prevention in atrial fibrillation: time to switch? Int J Clin Pract 67:603–605
Conolly SJ, Ezekowitz M, Yusuf S et al (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361:1139–1151
Tabata E, Yasaka M, Wakugawa Y et al (2013) Increase in the Size of an Intracardiac Thrombus during Dabigatran Therapy (110 mg b.i.d.) in an Acute Cardioembolic Stroke Patient. Cerebrovasc Dis Extra 3:78–80
Faust AC, Smith MB, Kawalsky D (2013) Acute ischemic stroke following cardioversion in a patient receiving dabigatran. Ann Pharmacother 47:1368–1371
Conway SE (2014) Fatal dabigatran-associated bleeding. Am J Health Syst Pharm 71:360
Ramos-Esquivel A, Salazar-Sánchez L (2013) Non-therapeutic anti-Xa levels in medical patients receiving anticoagulant therapy with enoxaparin. Thromb Res 132:433–436
Gosselin R, Hawes E, Moll S et al (2014) Performance of various laboratory assays in the measurement of dabigatran in patients receiving therapeutic doses: a prospective study based on peak and trough plasma levels. Am J Clin Pathol 141:262–267
Ingelheim Boehringer (2011) Pradaxa® Summary of Product Characteristics. Boehringer Ingelheim Pharmaceuticals Inc, CT
van Ryn J, Stangier J, Haertter S et al (2010) Dabigatran etexilate – a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 103:1116–1127
Ferreira J, Ferreira D, Viana-Baptista M et al (2012) Dabigatran for Stroke Prevention in Nonvalvular Atrial Fibrillation: answers to Challenging “Real-World” Questions. Thrombosis 2012:867121
Stangier J, Feuring M (2012) Using the HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis 23:138–143
Hankey GJ, Eikelboom JW (2011) Dabigatran Etexilate. A New Oral Thrombin Inhibitor. Circulation 123:1436–1450
Curvers J, van de Kerkhof D, Stroobants AK et al (2012) Measuring Direct Thrombin Inhibitors With Routine and Dedicated Coagulation Assays. Which Assay Is Helpful? Am J Clin Pathol 138:551–558
Harinstein LM, Morgan JW, Russo N (2013) Treatment of dabigatran-associated bleeding: case report and review of the literature. J Pharm Pract 26:264–269
Antovic JP, Skeppholm M, Eintrei J et al (2013) Evaluation of coagulation assays versus LC-MS/MS for determinations of dabigatran concentrations in plasma. Eur J Clin Pharmacol 69:1875–1881
Hapgood G, Butler J, Malan E et al (2013) The effect of dabigatran on the activated partial thromboplastin time and thrombin time as determined by the Hemoclot thrombin inhibitor assay in patient plasma samples. Thromb Haemost 110:308–315
Huisman MV, Lip GYH, Diener H-C et al (2012) Dabigatran etexilate for stroke prevention in patients with atrial fibrillation: resolving uncertainties in routine practice. Thromb Haemost 107(5):838–847
Reilly PA, Lehr T, Haertter S et al (2014) The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J Am Coll Cardiol 63:321–328
Acknowledgments
This study was supported by project APVV (Slovak Research and Development Agency) 0222-11 and by research project of Slovak Society of Cardiology 2012–2015.
Conflict of interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samoš, M., Stančiaková, L., Ivanková, J. et al. Monitoring of dabigatran therapy using Hemoclot® Thrombin Inhibitor assay in patients with atrial fibrillation. J Thromb Thrombolysis 39, 95–100 (2015). https://doi.org/10.1007/s11239-014-1125-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-014-1125-y