Abstract
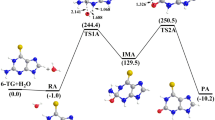
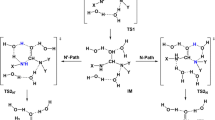
Mechanisms for the deamination of ammeline (AMN), guanine (Gua), and their analogues with nH2O (n = 1–3) have been investigated using B3LYP and G3MP2B3. The deamination reactions of AMN and Gua with 3H2O/OH−, and protonated AMN and Gua with 3H2O, were investigated using DFT. The rationale behind this work was to compare the deamination reactions of AMN and Gua analogues. Optimized geometries of the reactants, transition states, intermediates, and products were determined at B3LYP/6-31G(d,p) level of theory, and solvent calculations were performed using the solvation model on density (SMD). Thermodynamic properties (ΔH, and ΔG), proton affinities (PAs), gas-phase basicities (GBs), deprotonation enthalpies (Δacid H), and gas-phase acidities (Δacid G) were also calculated. For the SN2 mechanism, deamination can proceed via two possible pathways involving either two- or three-stepwise mechanisms producing a tetrahedral intermediate via four-, six-, or eight-membered transition states. The lowest overall activation energies of 128 (131) and 131 (133) kJ mol−1 at B3LYP/6-31G(d,p) in the gas phase (SMD) were obtained for the deamination of AMN and Gua with 3H2O, whereas the values of 140 (115) and 139 (117) kJ mol−1 were obtained for AMN and Gua with 3H2O/OH−, respectively. For protonated AMN and Gua with 3H2O in the gas phase (SMD), the overall activation energies are 233 (155) and 240 (162) kJ mol−1, respectively.













Similar content being viewed by others
References
Seffernick JL, Dodge AG, Sadowsky MJ, Bumpus JA, Wackett LP (2010) Bacterial ammeline metabolism via guanine deaminase. J Bacteriol 192:1106–1112
Jang YH, Hwang S, Chang SB, Ku J, Chung DS (2009) Acid dissociation constants of melamine derivatives from density functional theory calculations. J Phys Chem A 113:13036–13040
Lotsch BV, Schnick W (2006) Synthesis and structural characterization of the ammelinium salts [C3H6N5O]Cl, [C3H6N5O]Br, and [C3H6N5O]NO3. Z Anorg Allg Chem 632:1457–1464
Braekevelt E, Lau BY, Feng S, Ménard C, Tittlemier S (2011) Determination of melamine, ammeline, ammelide, and cyanuric acid in infant formula purchased in Canada by liquid chromatography-tandem mass spectrometry. Food Addit Contam 28:698–704
Brown CA, Jeong KS, Poppenga RH, Puschner B, Miller DM, Ellis AE, Kang KI, Sum S, Cistola AM, Brown SA (2007) Outbreaks of renal failure associated with melamine and cyanuric acid in dogs and cats in 2004 and 2007. J Vet Diagn Investig 19:525–531
Neidle S (1999) Oxford handbook of nucleic acid structure. Oxford University Press, Oxford
Gorb L, Kaczmarek A, Gorb A, Sadlej AJ, Leszczynski J (2005) Thermodynamics and kinetics of intramolecular proton transfer in guanine, post Hartree-Fock study. J Phys Chem B 109:13770–13776
Kim J, Park SI, Ahn C, Kim H, Yim J (2009) Guanine deaminase functions as dihydropterin deaminase in the biosynthesis of aurodrosopterin, a minor red eye pigment of Drosophila. J Biol Chem 284:23426–23435
Bitra A, Biswas A, Anand R (2013) Structural basis of the substrate specificity of cytidine deaminase superfamily Guanine deaminase. Biochemistry 52:8106–8114
Strazewski P (1988) Mispair formation in DNA can involve rare tautomeric forms in the template. Nucleic Acids Res 16:9377–9398
Yao L, Cukier RI, Yan H (2007) Catalytic mechanism of guanine deaminase: an ONIOM and molecular dynamics study. J Phys Chem B 111:4200–4210
Rayat S, Wu Z, Glaser R (2004) Nitrosative guanine deamination: ab initio study of deglycation of N-protonated 5-cyanoimino-4-oxomethylene-4 5-dihydroimidazoles. Chem Res Toxicol 17:1157–1169
Jin L, Wang W, Hu D, Lü J (2013) The conversion of protonated cytosine-SO3− to uracil-SO3−: insights into the novel induced hydrolytic deamination through bisulfite catalysis. Phys Chem Chem Phys 15:9034–9042
Jin L, Wang W, Hu D, Lü J (2014) A new insight into the 5-carboxycytosine and 5-formylcytosine under typical bisulfite conditions: a deamination mechanism study. Phys Chem Chem Phys 16:3573–3585
Glaser R, Wu H, Lewis M (2005) Cytosine catalysis of nitrosative guanine deamination and interstrand cross-link formation. J Am Chem Soc 127:7346–7358
Kazemi M, Åqvist J (2015) Chemical reaction mechanisms in solution from brute force computational Arrhenius plots. Nat Commun 6(7293):1–7
Hall RS, Fedorov AA, Marti-Arbona R, Fedorov EV, Kolb P, Sauder JM, Burley SK, Shoichet BK, Almo SC, Raushel FM (2010) The hunt for 8-oxoguanine deaminase. J Am Chem Soc 132:1762–1763
Almatarneh MH, Abus-Saleh AA, Uddin KM, Poirier RA, Warburton PL (2016) A computational mechanistic study of the deamination reaction of melamine. Int J Quantum Chem 117:180–189
Uddin KM, Flinn CG, Poirier RA, Warburton PL (2014) Comparative computational investigation of the reaction mechanism for the hydrolytic deamination of cytosine butane dimer and 5,6-saturated cytosine analogues. Comp Theor Chem 1027:91–102
Alrawashdeh AI, Almatarneh MH, Poirier RA (2013) Computational study on the deamination reaction of adenine with OH−/nH2O (n= 0, 1, 2, 3) and 3H2O. Can J Chem 91:518–526
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G (2009) Gaussian 09, Revision A.02, Gaussian Inc., Wallingford CT
Uddin KM, Almatarneh MH, Shaw DM, Poirier RA (2011) Mechanistic study of the deamination reaction of guanine: a computational study. J Phys Chem A 115:2065–2076
Uddin KM, Poirier RA (2011) Computational study of the deamination of 8-oxoguanine. J Phys Chem B 115:9151–9159
Almatarneh MH, Flinn CG, Poirier RA, Sokalski WA (2006) Computational study of the deamination reaction of cytosine with H2O and OH−. J Phys Chem A 110:8227–8234
Glasstone S, Laidler K, Eyring H (1941) The theory of rate processes, 1st edn. McGraw Hill, New York
Benson SW (1969) The foundations of chemical kinetics. McGraw-Hill, New York
Ehrlich M, Norris KF, Wang RY-H, Kuo KC, Gehrke CW (1986) DNA cytosine methylation and heat-induced deamination. Biosci Rep 6:387–393
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Mukherjee S, Ren J (2010) Gas-phase acid-base properties of melamine and cyanuric acid. J Am Soc Mass Spectrom 21:1720–1729
Zhachkina A, Liu M, Sun X, Amegayibor FS, Lee JK (2009) Gas-phase thermochemical properties of the damaged base O6-methylguanine versus adenine and guanine. J Org Chem 74:7429–7440
Yu Y, Liu K, Zhao H, Song D (2013) Mechanism of the deamination of isoguanine: a theoretical investigation. J Phys Chem A 117:5715–5725
Acknowledgements
We are grateful to the Natural Sciences and Engineering Council of Canada (NSERC) for financial support. We also would like to thank Compute Canada and the Atlantic Computational Excellence Network (ACENET) for computer time.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Human and animal rights
There is no research involving Human Participants and/or Animals.
Additional information
This paper is dedicated to Professor Lou Massa on the occasion of his Festschrift: A Path through Quantum Crystallography.
Electronic supplementary material
Full geometries and energies of all molecules studied are reported.
ESM 1
(DOCX 3644 kb)
Rights and permissions
About this article
Cite this article
Uddin, K.M., Henry, D.J., Alrawashdeh, A.I. et al. Mechanism for the deamination of ammeline, guanine, and their analogues. Struct Chem 28, 1467–1477 (2017). https://doi.org/10.1007/s11224-017-0941-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-017-0941-z