Abstract
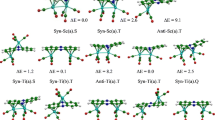
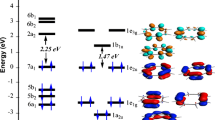
Geometry optimizations have been performed on the M4(Pyr)2 (M = Ti-Ni, Pd and Pt, Pyr = C16H10) complexes by means of DFT method using BP86 and mPW1PW91 functionals combined to the TZP basis set. The M4 moiety encapsulated between two pyrene ligands tends to establish M-L bonding with various hapticies from η2 to η6. In accordance with the coordination modes, the pyrene behaves as neutral, dianionic, or tetraanionic ligand. For the Ti, V, and Fe, the low-spin (S = 0) and the high-spin (S = 1) structures are isoenergetic, while the Cr, Mn, and Co structures prefer the high-spin states. The Ni, Pd, and Pt structures are more favorable in low-spin state. The zigzag metallic chain is predicted to be more stable than that of the two-dimensional sheet for the Pd complexes. The spin state changes of the studied complexes in their ground states could be characterized in some cases by different molecular structure modifications (structural isomerisation, where structural modifications accompany the spin state modification like as bonds and angles), electronic configurations (low-spin or high-spin), or oxidation states with respect to the metal charges, in agreement with the metal nature. The optimized structures obtained by both BP86 and mPW1PW91 methods are consistent to each other, where the energetic parameters follow similar tendencies regarding the stability order between isomers.







Similar content being viewed by others
References
Debad JD, Morris JC, Lynch V, Magnus P, Bard AJ (1996) J Am Chem Soc 118:2374
Harvey RG (1997) Polycyclic aromatic hydrocarbons. Wiley VCH, New York
Ohashi K, Kubo T, Masui T, Yamamoto K, Nakasuji K, Takui T, Kai Y, Murata I J Am Chem Soc
Shibasaki T, Komine N, Hirano M, Komiya S (2006) Organometallics 25:523
Bendjaballah S, Kahlal S, Costuas K, Be Villon E, Saillard J-Y (2006) Chem-Eur J 12:2048
Korichi H, Zouchoune F, Zendaoui S-M, Zouchoune B, Saillard J-Y (2010) Organometallics 29:1693
Farah S, Ababsa S, Benhamada N, Zouchoune B (2010) Polyhedron 29:2722
Bouchakri N, Benmachiche A, Zouchoune B (2011) Polyhedron 30:2644
Benmachiche A, Zendaoui SM, Bouaoud SE, Zouchoune B (2012) Electronic structure and coordination chemistry of phenanthridine ligand in first-row transition metal complexes: A DFT study. Int J Quant Chem 113(7):985-996
Chekkal F, Zendaoui SM, Zouchoune B, Saillard J-Y (2013) New J Chem 37:2293
Merzoug M, Zouchoune B (2014) J Organomet Chem 770:69
Zendaoui SM, Zouchoune B (2013) Polyhedron 51:123
Murahashi T, Inoue R, Isui K, Ogoshi S (2009) J Am Chem Soc 131:9888
T. Murahashi, N. Kato, T. Uemura and H. Kurosawa, Angew. Chem.Int, Ed., 46, 3509 (2007).
Tatsumi T, Shirato K, Murahashi T, Ogoshi S, Kurosawa H (2006) Angew Chem 118:5931
Murahashi T, Uemura T, Kurosawa H (2003) J Am Chem Soc 125:8436
Ceccon A, Santi S, Orian L, Bisello A (2004) Coord Chem Rev 248:683
Manriquez JM, Ward MD, Reiff WM, Calabrese JC, Jones NL, Carroll PJ, Bunel EE, Miller JS (1995) J Am Chem Soc 117:6182
Esponda E, Adams C, Burgos F, Chavez I, Manriquez JM, Delpech F, Castel A, Gornitzka H, Rivière-Baudet M, Rivière P (2006) J Organomet Chem 691:3011
Noodleman L, Han W-G (2006) J Biol Inorg Chem 11:674
Cremer D (2001) Mol Phys 99:1899
Löwdin PO (1955) Phys Rev 97:1509
Lykos P, Pratt GW (1963) Rev Mod Phys 35:496
Yamaguchi K (1990) Self-consistent field: theory and applications. In: Carbo R, Klobukowski M (eds) . Elsevier, Amsterdam, p. 727
Yamaguchi K, Kawakami T, Takano Y, Kitagawa Y, Yamashita Y, Fujita H (2002) Int J Quantum Chem 90:370
Hehre WJ, Random L, von Schleyer PR, Pople JA (1986) Ab initio molecular orbital theory. New York, Wiley
Szabo A, Ostlund NS (1996) Modern quantum chemistry. New York, Dover Publications, Inc.
Murahashi T, Fujimoto M, Okao M, Hashimoto Y, Uemura T, Tatsumi Y, Nakao Y, Ikeda A, Sakaki S, Kurosawa H (2006) Science 313:1104
Zouchoune F, Zendaoui S-M, Bouchakri N, Djedouani A, Zouchoune B (2010) J Mol Struct 945:78
Farah S, Korichi H, Zendaoui SM, Saillard JY, Zouchoune B (2009) Inorg Chim Acta 362:3541
Peng A, Zhang X, Li QS, King RB, Scharfer III HF (2013) New J Chem 37:775
Wang H, King RB, Schaefer III HF (2008) Eur J Inorg:3698
Fan Q, Feng H, Sun W, Li H, Xie Y, King RB, Scharfer III HF (2013) New J Chem 37:1545
Peng A, Zhang X, Li QS, King RB, Schaefer III HF (2013) New J Chem 37(775)
ADF2014.01, Theoretical Chemistry, Vrije Universiteit: Amsterdam, The Netherlands, SCM
Baerends EJ, Ellis DE, Ros P (1973) Chem Phys 2:41
te Velde G, Baerends EJ (1992) J Comput Phys 99:84
Fonseca Guerra C, Snijders JG, te Velde G, Baerends EJ (1998) Theo Chim Acc 99:391
Bickelhaupt FM, Baerends EJ (2000) Rev Comput Chem 15:1
te Velde G, Bickelhaupt FM, Fonseca Guerra C, van Gisbergen SJA, Baerends EJ, Snijders JG, Ziegler T (2001) J Comput Chem 22:931
Vosko SD, Wilk L, Nusair M (1990) Can J Chem 58:1200
Becke AD (1986) J Chem Phys 84:4524
Becke AD (1988) Phys Rev A 38:3098
Perdew JP (1986) Phys Rev B 33:8822
Perdew JP (1986) Phys Rev B 34:7406
Adamo C, Barone V (1998) J Chem Phys 108:664
van Lenthe E, Ehlers AW, Bearends EJ (1999) J Chem Phys 110:8943
Versluis L, Ziegler T (1988) J Chem Phys 88:322
Fan L, Ziegler T (1992) J Chem Phys 96:9005
Fan L, Ziegler T (1992) J Phys Chem 96:6937
P. Flükiger, H. P. Lüthi, S. Portmann, J. Weber, MOLEKEL, Version 4.3.win32 Swiss Center for Scientific Computing (CSCS), Switzerland, 2000–2001. http://www.cscs.ch/molekel/.
Weinhold F, Landis CR (2005) Valency and bonding: a natural bond order donor–acceptor perspective. Cambridge University Press, U. K.
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F (2001) Natural Bond Orbitals “Analysis Programs” Theoretical Chemistry Institute. University of Wisconsin, Madison, WI
B. Eliasson, T. Lejon, U. Edlund (1984) J Chem Soc Chem Commun 591
Miillen K (1978) Helv Chim Acta 61:2307
Dahl LF, Rundle RE (1963) Acta Cryst 16:419
Wang H, Sun Z, Xie Y, King RB, Schaefer III HF (2011) Inorg Chem 50:9256
Kitagawa Y, Saito T, Ito M, Shoji M, Koizumi K, Yamanaka S, Kawakami T, Okumura M, Yamaguchi K (2007) Chem Phys Lett 442:445
Kitagawa Y, Saito T, Nakanishi Y, Kataoka Y, Matsui T, Kawakami T, Okumura M, Yamaguchi K (2009) J Phys Chem A 113:15041
Minsky A, Klein J, Rabinovitz M (1981) J Am Chem Soc 103:4586
Farah S, Bouchakri N, Zendaoui SM, Saillard JY, Zouchoune B (2010) J Mol Struct 953:143
A. Saiad, B. Zouchoune. Can. J. Chem., 93, 1096 (2015).
S. Aduldecha, B. Hathaway (1991) J Chem Soc Dalton Trans 993
S.-J. Shieh, C.-C. Chou, G.-H. Lee, C.-C. Wang, S.-M. Peng (1997) Angew Chem 109: 57; Angew. Chem. Int. Ed. Engl 36:56.
Peng S-M, Wang C-C, Jang Y-L, Chen Y-H, Li F-Y, Mou C-Y, Leung M-K (2000) J Magn Magn Mater 209:80
López X, Huang M-Y, Huang GC, Peng SM, Li FY, Bénard M, Rohmer M-M (2006) Inorg Chem 45:9075
Bera JK, Dunbar KR (2002) Angew Chem Int Ed 41:4453
Philpott MR, Kawazoe Y (2007) Chem Phys 337:55
Murahashi T, Mochizuki E, Kai Y, Kurosawa H (1999) J Am Chem Soc 121:10 660
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
Bond distances (Å), bond angles (°), and the total bonding energies (eV) for the optimized geometries of the computed compounds in their different spin states and various symmetries are given in Tables S1–S6. BP86-optimized structures are given in Figs. S1–S3, values and plots of spin densities are given in Schemes S1–S3, and MO diagram of Pd4(Pyr)2 is given in Scheme S4. (DOCX 2595 kb)
Rights and permissions
About this article
Cite this article
Fadli, S., Zouchoune, B. Coordination chemistry and bonding analysis of tetranuclear transition metal pyrene sandwich complexes. Struct Chem 28, 985–997 (2017). https://doi.org/10.1007/s11224-016-0905-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-016-0905-8