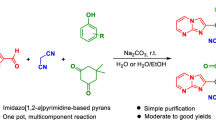
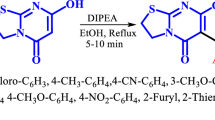
Abstract
In this work, an efficient isocyanide-based three component method is developed for the synthesis of the novel substituted pyrano[2,3-d][1,3,4]thiadiazolo[3,2-a]pyrimidine system, having both the biologically active pyranopyrimidine and thiadiazolopyrimidine scaffolds, from the readily attainable reaction of 7-hydroxy-2-phenyl-5H-[1,3,4]thiadiazolo[3,2-a]pyrimidine-5-one, isocyanides, and dialkylacetylenedicarboxylates. This procedure has the chief advantages of fairly high reaction yields, mild reaction conditions, high atom-economy, and catalyst-free reaction.
Graphical Abstract






Similar content being viewed by others
Notes
Single-Crystal X-ray data for 4a (CCDC 151190): C24H24N4O6S: MW = 496.53, Monoclinic, space group P21, cell dimensions a = 10.1483 (8) Å, b = 17.5745 (14) Å, c = 12.6336 (11) Å, β = 93.342 (1)°, V = 2249.4 (3) Å3, Z = 4, Dx = 1.466 Mg m−3, F000 = 1040, crystal size 0.32 × 0.31 × 0.31 mm, Crystallographic data were collected on a Bruker Smart APEX CCD diffractometer Kα radiation (λ = 0.71073 Å). A total of 43,168 reflections (2 ≤θ ≤ 29.4) were collected at a temperature of 100(2) K in a series of ω scans in 1° oscillations and integrated using the Stoe X-AREA software package (5031 reflections were unique with I > 2σ(I)). The structure was solved by direct method and subsequent different Fourier maps and then refined on F2 by a full-matrix least-square procedure using anisotropic displacement parameters. All non-H atoms were refined anisotropically and H-atoms were placed in the ideal positions. Final residuals were R = 0.039 and Rw = 0.
Single-Crystal X-ray data for 4d (CCDC 1524054): C28H34N4O6S: MW = 554.65, Monoclinic, space group P21, cell dimensions a = 8.2682 (3) Å, b = 20.2332 (7) Å, c = 17.1811 (6) Å, α = 90 °, β = 102.056(1)°, γ = 90 °, V = 2810.87(17) Å3, Z = 4, Dx = 1.311 Mg m−3, F000 = 1176 , crystal size 0.194 × 0.094 × 0.083 mm, Crystallographic data were collected on a Bruker D8 VENTURE PHOTON 100 CMOS diffractometer with graphite monochromated Cu Kα radiation (λ = 1.54178 Å). A total of 44,187 reflections (5.2651 ≤ θ ≤ 74.5595) were collected at a temperature of 150(2) K in a series of ω scans in 1° oscillations and integrated using the Stoe X-AREA software package (5031 reflections were unique with I > 2σ(I)). The structure was solved by direct method and subsequent different Fourier maps and then refined on F2 by a full-matrix least-square procedure using anisotropic displacement parameters. All non-H atoms were refined anisotropically and H-atoms were placed in the ideal positions. Final residuals were R = 0.0398 and Rw = 0.0979 for 5736 parameters.
References
C.V. Galliford, K.A. Scheidt, Angew. Chem. Int. Ed. 46, 8748 (2007)
K. Kumar, H. Waldmann, Angew. Chem. Int. Ed. 48, 3224 (2009)
A.L. Harvey, Curr. Opin. Chem. Biol. 11, 480 (2007)
D.J. Newman, G.M. Cragg, K.M. Snader, J. Nat. Prod. 66, 1022 (2003)
R. Breinbauer, I.R. Vetter, H. Waldman, Angew. Chem. Int. Ed. 41, 2879 (2002)
A.M. Boldi, Curr. Opin. Chem. Biol. 8, 281 (2004)
B.B. Toure, D.G. Hall, Chem. Rev. 109, 4439 (2009)
J.K. Erguden, H.W. Moore, Org. Lett. 1, 375 (1999)
G. Raffa, M. Rusch, G. Balme, N. Monteiro, Org. Lett. 11, 5254 (2009)
J. An, L.-Q. Lu, Q.-Q. Yang, T. Wang, W.-J. Xiao, Org. Lett. 15, 542 (2013)
S.J. Gharpure, P. Niranjana, S.K. Porwal, Org. Lett. 14, 5476 (2012)
H.Y. Lee, Y. Jung, Y. Yoon, B.G. Kim, Y. Kim, Org. Lett. 12, 2672 (2010)
K.M. Brummond, D. Chen, M.M. Davis, J. Org. Chem. 73, 5064 (2008)
N.T. Patil, R.D. Kavthe, V.S. Raut, V.S. Shinde, B. Sridhar, J. Org. Chem. 75, 1277 (2010)
N.S. El-Sayed, E.R. El-Bendary, S.M. El-Ashry, M.M. El-Kerdawy, Eur. J. Med. Chem. 46, 3714 (2011)
R. Lin, S.G. Johnson, P.J. Connolly, S.K. Wetter, E. Binnun, T.V. Hughes, W.V. Murray, N.B. Moreno-Mazza, S.J. Adams, M. Fuentes- Pesquera, A.R. Middleton, S.A. Pandey, Bioorg. Med. Chem. Lett. 19, 2333 (2009)
S. Yokohama, T. Miwa, S. Aibara, H. Fujiwara, H. Matsumoto, K. Nakayama, T. Iwamoto, M. Mori, R. Moroi, W. Tsukada, S. Isoda, Chem. Pharm. Bull. 40, 2391 (1992)
A.K. Gadad, C.S. Mahajanshetti, S. Nimbalkar, A. Raichurkar, Eur. J. Med. Chem. 35, 853 (2000)
S.S. Shukurov, M.A. Kukaniev, I.M. Nasyrov, K.S. Zakharov, R.A. Karakhanov, Russ. Chem. Bull. 11, 1871 (1993)
B.H. Lee, M.F. Clothier, F.E. Dutton, George A. Conder, S.S. Johnson, Bioorg. Med. Chem. Lett. 8, 3317 (1998)
L.P. DuanA, Q.F. Zhao, B, H.B. Zhang. Arab. J. Chem. 3, 225 (2010)
C.W. Sun, X.D. Zhang, H.P. Zhou, Bioorg. Med. Chem. Lett. 14, 8574 (2006)
Brian W. Clare, Claudiu T. Supuran, Eur. J. Med. Chem. 35, 859 (2000)
M. Suiko, E. Taniguchi, K. Maekava, M. Etoagric, Biol. Chem. 43, 741 (1979)
A.A. Stierle, D.B. Stierle, T. Girtsman, T.C. Mou, C. Antczak, H. Djaballah, J. Nat. Prod. 78, 2917 (2015)
J. Kozlowski, C. Coburn, W. Yu, L. Tong, B. Hu, B. Zhong, J. Hao, D. Wang, T. Ji, WO Patent Application 2015094998 A1 (2015)
H. Huang, X. Feng, Z. Xiao, L. Liu, H. Li, L. Ma, Y. Lu, J. Ju, Z. She, Y. Lin, J. Nat. Prod. 74, 997 (2011)
Z. Sui, X. Zhang, X. Li, WO Patent Application 2006047017 A1 (2006)
N. Panthama, S. Kanokmedhakul, K. Kanokmedhakul, K. Soytong, J. Nat. Prod. 74, 2395 (2011)
J.-M. Gao, S.-X. Yang, J.-C. Qin, Chem. Rev. 113, 4755 (2013)
E.M. Grivaky, S. Lee, J. Med. Chem. 23, 327 (1980)
J.A. Valderrama, P. Colonelli, D. Väsquez, M.F. Gonzälez, J.A. Rodriguez, C. Theoduloz, Bioorg. Med. Chem. 16, 10172 (2008)
M.C. Bagley, D.D. Hughes, M.C. Lubinu, E.A. Merrit, P.H. Taylor, N.C.O. Tomkinson, QSAR Combust. Sci. 23, 859 (2004)
S. Furuya, T. Ohtaki, Chem. Abstr. 121, 205395 (1994)
N.R. Kamdar, D.D. Haveliwala, P.T. Mistry, S.K. Patel, Eur. J. Med. Chem. 45, 5056 (2010)
H.M. Aly, M.M. Kamal, Eur. J. Med. Chem. 47, 18 (2012)
J. Hren, F. Pozgan, A. Bunic, V.I. Parvulescu, S. Polanc, M. Kocevar, Tetrahedron 65, 8216 (2009)
Multicomponent Reactions I; T. J. J. Müller, Science of Synthesis, Georg ThiemeKG: Stuttgart, New York, (2014)
A. Shaabani, A. Sarvary, A. Maleki, Wiley-VCH: Weinheim, (2012)
A. Dömling, Chem. Rev. 106, 17 (2006)
J.P. Zhu, Eur. J. Org. Chem. 1133 (2003)
V. Nair, C. Rajesh, A.U. Vinod, S. Bindu, A.R. Sreekanth, J.S. Mathen, L. Acc, Balagopal. Chem. Res. 36, 899 (2003)
A. Dömling, I. Ugi, Angew. Chem. Int. Ed. 39, 3168 (2000)
C. Hulme, V. Gore, Curr. Med. Chem. 10, 51 (2003)
Multicomponent Reactions in Organic Synthesis; J. Zhu, Q. Wang, M.X. Wang, Weinheim, Wiley-VCH (2015)
S. Lang, Chem. Soc. Rev. 42, 4867 (2013)
G. Qiu, Q. Ding, Wu. J. Chem. Soc. Rev. 42, 5257 (2013)
T. Vlaar, E. Ruijter, B.U. Maes, R.V. Orru, Angew. Chem. Int. Ed. 52, 7084 (2013)
A.V. Lygin, A. de Meijere, Angew. Chem. Int. Ed. 49, 9094 (2010)
S. Sadjadi, M.M. Heravi, Tetrahedron 67, 2707 (2011)
A. Shaabani, R. Ghadari, A. Sarvary, A.H. Rezayan, J. Org. Chem. 74, 4372 (2009)
A.A. Esmaeili, M. Darbanian, Tetrahedron 59, 5545 (2003)
N. Sharma, Z. Li, U.K. Sharma, E.V. Van der Eycken, Org. Lett. 16, 3884 (2014)
X. Wang, S.Y. Wang, S. Ji, J. Org. Lett. 15, 1954 (2013)
M.A. Terzidis, J. Stephanidou-Stephanatou, C.A. Tsoleridis, J. Org. Chem. 75, 1948 (2010)
T. Fang, Q. Tan, Z. Ding, B. Liu, B. Xu, Org. Lett. 16, 2342 (2014)
Y. Tian, L. Tian, X. He, C. Li, X. Jia, J. Li, Org. Lett. 17, 4874 (2015)
S. Jia, S. Su, C. Li, X. Jia, J. Li, Org. Lett. 16, 5604 (2014)
F. Sha, L. Wu, X. Huang, J. Org. Chem. 77, 3754 (2012)
V. Nair, R.S. Menon, P.B. Beneesh, V. Sreekumar, S. Bindu, Org. Lett. 6, 767 (2004)
I. Yavari, M.T. Maghsoodlou, J. Chem. Res. 386 (1998)
I. Yavari, M. Sirouspour, S. Souri, Mol. Divers. 10, 265 (2006)
M. Teimouri, B.R. Bazhrang, V. Eslamimanesh, A. Nouri, Tetrahedron 62, 3016 (2006)
A. Shaabani, R. Ghadari, A. Sarvary, A.H. Rezayan, J. Org. Chem. 74, 4372 (2009)
A. Shaabani, E. Soleimani, A. Sarvary, A.H. Rezayan, Bioorg. Med. Chem. Lett. 18, 3968 (2008)
M.T. Maghsoodlou, I. Yavari, F. Nassiri, H. Djahaniani, Z. Razmjoo, Monatsh. Chem. 134, 1585 (2003)
A. Shaabani, A. Sarvary, A.H. Rezayan, S. Keshipour, Tetrahedron 65, 3492 (2009)
K. Rad-Moghadam, A. Taghizadeh Valadi, A. Alipour, Appl. Organometal. Chem. 28, 146 (2014)
M.B. Teimouri, R. Bazhrang, Monatsh. Chem. 140, 513 (2009)
R. Akbarzadeh, T. Amanpour, P. Mirzaei, A. Bazgir, Helv. Chim. Acta 95, 483 (2012)
T. Amanpour, P. Mirzaei, A. Bazgir, Synthesis 2, 235 (2012)
A. Hassanabadi, M.H. Mosslemin, M. Anary-Abbasinejad, M. Ghasemi, Synth. Commun. 41, 3714 (2011)
M. Anary-Abbasinejad, H. Anaraky-Ardakani, F. Rastegari, A. Hassanabadi, J. Chem. Res. 10, 602 (2007)
M. Zangouei, A.A. Esmaeili, A. Habibi, A. Fakhari, Tetrahedron 70, 8619 (2014)
A.A. Esmaeili, F. Salehan, A. Habibi, A.R. Fakhari, Tetrahedron Lett. 57, 100 (2016)
A.A. Esmaeili, S. Amini-Ghalandarabad, F. Mesbah, M. Tasmimi, M. Izadyar, A.R. Fakhari, A.R. Salimi, Tetrahedron 71, 2458 (2015)
A.A. Esmaeili, M. Zangouei, A.R. Fakhari, A. Habibi, Tetrahedron Lett. 53, 1351 (2012)
N. Suzuki, T. Miwa, S. Aibara, H. Kanno, H. Takamori, M. Tsubokawa, Y. Ryokawa, W. Tsukada, S. Isoda, Chem. Pharm. Bull. 40, 357 (1992)
Acknowledegements
The Research Council of Ferdowsi University of Mashhad is acknowledged for financial support (Grant No. 3/41156).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Akhavan, M., Esmaeili, A.A., Zangouei, M. et al. Convenient one-pot access to novel densely functionalized pyrano[2,3-d][1,3,4]thiadiazolo[3,2-a]pyrimidines via three component reaction. Res Chem Intermed 43, 4683–4696 (2017). https://doi.org/10.1007/s11164-017-2904-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-2904-9