Abstract
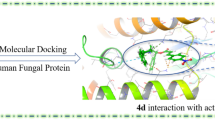
A computational Petra/Osiris/Molinspiration and Density Functional Theory based model has been developed for the identification of physic–chemical parameters governing the bioactivity of chiral amides derivatives of diacetyl-L-tartaric acid and aromatic amines 4–9 containing combined antifungal pharmacophore sites. The six compounds 4–9 analyzed here were previously experimentally and now virtually screened for their antibacterial/antifungal activity. The highest antifungal activity was obtained for compound 6, which exhibited excellent % inhibition, comparable to Terbinafine. Compound 5, represents increased activity as compared to its isomer 6. The increase of bioactivity from 5 to 6 could be attributed to the existence of pi-charge transfer from para-Bromo-phenyl to its amid group (COδ−--NHδ+), which plays a crucial template role in the organization of antifungal O,O-phramacophore sites. Moreover, it is cheap, has fewer side effects, and its possible inclusions in selective fungal/viral media such as Fusarium, HIV, and Hepatitis B/C have to be questioned.










Similar content being viewed by others
References
M.R.P. Joseph, A.M. Al-Hakami, M.M. Assiry, A.S. Jamil, A.M. Assiry, M.A. Shaker, M.E. Hamid, J. Mycol. Med. 25, 17 (2015)
V. Aiassa, A. Zoppi, I. Albesa, M.R. Longhi, Carbohydr. Polym. 121, 320 (2015)
L. Dendooven, P. Splatt, J.M. Anderson, Soil Biol. Biochem. 26, 925 (1994)
http://www.pharma.us.novartis.com/product/pi/pdf/Lamisil_tablets.pdf
M. Malik, S.W. Khan, M. Arfan, J.H. Zaidi, A. Bano, F. Ullah, Asian J. Chem. 25, 745 (2013)
M.S. Hossain, M.A. Hossain, M.M. Rahman, M.A.M. Mondol, M.S.A. Bhuiyan, A.I. Gray, M.E. Flores, M.A. Rashid, Phytochem. 65, 2147 (2004)
N.S. Vatmurge, B.G. Hazra, V.S. Pore, F. Shirazi, P.S. Chavan, M.V. Deshpande, Bioorganic Med. Chem. Lett. 18, 2043 (2008)
C. Landon, P. Sodano, C. Hetru, J. Hoffmann, M. Ptak, Protein Sci. 6, 1878 (1997)
R.G. Bartsch, G.L. Newton, C. Sherrill, R.C. Fahey, J. Bacteriol. 178, 4742 (1996)
B.A. Kundim, Y. Itou, Y. Sakagami, R. Fudou, S. Yamanakac, M. Ojikaa, Tetrahedron 60, 10217 (2004)
G.K. Liyanage, F.J. Schmitz, J. Nat. Prod. 59, 148 (1996)
A.D. Becke, J. Chem. Phys. 98, 5648 (1993)
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B 37, 785 (1988)
J. Sheikh, K. Hatzade, A. Bader, U. Shaheen, T. Sander, T. Ben, Hadda. Med. Chem. Res. 23, 243 (2014)
T.B. Hadda, S. Srivastava, B. Das, H. Salgado-Zamora, U. Shaheen, A. Bader, M.M. Naseer, Med. Chem. Res. 23, 995 (2014)
T.B. Hadda, M.A. Ali, V. Masand, S. Gharby, T. Fergoug, I. Warad, Med. Chem. Res. 22, 1438 (2013)
T.B. Hadda, T. Fergoug, I. Warad, Res. Chem. Intermed. 39, 1963 (2013)
J. Fathi, V. Masan, R. Jawarkar, R. Mouhoub, T.B. Hadda, J. Comput. Method Mol. Design 1, 57 (2011)
A. Jarrahpour, J. Fathi, M. Mimouni, T.B. Hadda, J. Sheikh, Z.H. Chohan, A. Parvez, Med. Chem. Res. 21, 1984 (2012)
A. Parvez, M. Jyotsna, M.H. Youssoufi, T.B. Hadda, Phosphorus Sulfur Silicon Relat. Elem. 185, 1500 (2010)
Z.H. Chohan, S.H. Sumrra, M.H. Youssoufi, T.B. Hadda, Eur. J. Med. Chem. 45, 2739 (2010)
Z.H. Chohan, M.H. Youssoufi, A. Jarrahpour, T.B. Hadda, Eur. J. Med. Chem. 45, 1189 (2010)
A. Jarrahpour, M. Motamedifar, M. Zarei, M.H. Youssoufi, M. Mimouni, Z.H. Chohan, T.B. Hadda, Phosphorus Sulfur Silicon Relat. Elem. 185, 491 (2010)
J. Sheikh, A. Parvez, V. Ingle, H. Juneja, R. Dongre, Z.H. Chohan, M.H. Youssoufi, T.B. Hadda, Eur. J. Med. Chem. 46, 1390 (2011)
Z.H. Chohan, S.H. Sumrra, M.H. Youssoufi, T.B. Hadda, J. Coord. Chem. 63, 3981 (2010)
Y.N. Mabkhot, A.M. Al-Majid, A. Barakat, S.S. Al-Showiman, M.S. Al-Har, S. Radi, M.M. Naseer, T.B. Hadda, Int. J. Mol. Sci. 15, 5115 (2014)
C.A. Lipinski, F. Lombardo, B.W. Dominy, P.J. Feeney, Adv. Drug Deliv. Rev. 46, 3 (2001)
C.A. Lipinski, Drug Discov. Today Technol. 1, 337 (2004)
T.B. Hadda, Z.K. Genc, V.H. Masand, N. Nebbache, I. Warad, S. Jodeh, M. Genc, Y.N. Mabkhot, A. Barakat, H. Salgado-Zamora, Acta Chim. Slov. 62, 679 (2015)
J. Fleming, Frontier Orbitals and Organic Chemical Reactions, vol. 111 (Wiley, London, 1976)
D. Sajan, K.U. Lakshmi, Y. Erdogdu, I.H. Joe, Spectrochim. Acta 78A, 113 (2011)
B. Eren, A. Unal, Spectrochim. Acta Part A 103, 222 (2013)
J. Olsen, P.J. Jorgensen, Chem. Phys. 82, 3235 (1985)
C. Alasalvar, M.S. Soylu, Y. Unver, G. Apaydın, D. Unluer, Spectrochim. Acta Part A 1033, 243 (2013)
Acknowledgments
The authors greatly acknowledge the Higher Education Commission of Pakistan for funding this research. T.B.H.and Y.N.M. would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this Research group N (PRG-1437-29).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mabkhot, Y.N., Arfan, M., Zgou, H. et al. How to improve antifungal bioactivity: POM and DFT study of some chiral amides derivatives of diacetyl-L-tartaric acid and amines. Res Chem Intermed 42, 8055–8068 (2016). https://doi.org/10.1007/s11164-016-2578-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2578-8