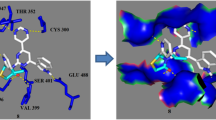
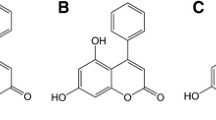
Abstract
The structure and antibacterial activity of previously synthesized and tested compounds 4-amino-5-hydroxy 2(5H)-furanones 2a–h have been investigated. These compounds have previously been screened against Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, and Pseudomonas aeruginosa ATCC 27853. Here we report results from POM analysis of their interaction with bacterial targets. The structure and governing force of the antibacterial pharmacophore site of furanones 2a–h were established on the basis of a hypothetical and semi-empirical antibacterial (Xδ+–Yδ−) pharmacophore site.
Graphical abstract
From structure–activity relationship studies, it seems that the functionalized moiety (HO-C-C-NH-R) of the five membered furanone ring and the supplementary halogen substituent are essential for the anti-bacterial activity of this series.




Similar content being viewed by others
Notes
Osiris molecular properties calculation software is available online at the site: www.osiris.com.
References
J. Coombs, E. Lattmann, H.M.R. Hoffmann, Synthesis 9, 1367 (1998)
P. Pantazis, Leuk. Res. 19, 775 (1995)
Q. Cai, J.R. Lindsey, R. Zhang, Int. J. Oncol. 10, 953 (1997)
E. Lattmann, S. Dunn, S. Niamsanit, N. Sattayasai, Bioorg. Med. Chem. Lett. 15, 919 (2005)
J. Gasteiger, Empirical Methods for the Calculation of Physicochemical Data of Organic Compounds, in: Physical Property Prediction in Organic Chemistry, Ed. C. Jochum, M. G. Hicks, J. Sunkel, (Springer, Heidelberg, 1988) p. 119
T.B. Hadda, B. Rahima, A. Kerbal, B.F. Baba, M. Akkurt, G. Demailly, M. Benazza, Arkivoc ii, 1 (2008)
T.B. Hadda, R. Badri, A. Kerbal, B.F. Baba, M. Akkurt, G. Demailly, M. Benazza, Arkivoc xiv, 276 (2007)
G.A. Houari, A. Kerbal, B. Bennani, M.F. Baba, M. Daoudi, T.B. Hadda, Arkivoc xii, 42 (2008)
T.B. Hadda, M. Akkurt, M.F. Baba, M. Daoudi, B. Bennani, A. Kerbal, Z.H. Chohan, J. Enz. Inhib. Med. Chem. 24, 457 (2009)
A. Parvez, M. Jyotsna, M.H. Youssoufi, T.B. Hadda, Phosphorus, Sulfur Silicon Related Elem. 185, 1500 (2010)
A. Jarrahpour, M. Motamedifar, M. Zarei, M.H. Youssoufi, M. Mimouni, Z.H. Chohan, T.B. Hadda, Phosphorus, Sulfur Silicon Related Elem. 185, 491 (2010)
Z.H. Chohan, M.H. Youssoufi, A. Jarrahpour, T.B. Hadda, Eur. J. Med. Chem. 45, 1189 (2010)
Z.H. Chohan, S.H. Sumrra, M.H. Youssoufi, T.B. Hadda, Eur. J. Med. Chem. 45, 2739 (2010)
A. Parvez, V. Tiwari, J. Sheikh, R. Dongre, M.H. Youssoufi, T.B. Hadda, Eur. J. Med. Chem. 45, 4370 (2010)
A. Parvez, J. Meshram, M.H. Youssoufi, T.B. Hadda, Phosphorus, Sulfur Silicon Related Elem. 17, 1500 (2010)
Z.H. Chohan, H.A. Shad, L. Toupet, T.B. Hadda, M. Akkurt, J. Chem. Crystallogr. (2010). doi:10.1007/s10870-010-9856-x
Z.H. Chohan, S.H. Sumrra, M.H. Youssoufi, T.B. Hadda, J. Coord. Chem. 63, 3981 (2010)
A. Parvez, J. Meshram, J. Sheikh, V. Tiwari, R. Dongre, T.B. Hadda, Eur. J. Med. Chem. 45, 4370 (2010)
R.D. Jawarkar, V.H. Masand, K.N. Patil, D.T. Mahajan, M.H. Youssoufi, T.B. Hadda, S.L. Kumbhare, Der Pharm. Chem. 2, 875 (2010)
B. Bennani, A. Kerbal, M. Daoudi, B.F. Baba, G.A. Houari, A. Jalbout, M. Mimouni, M. Benazza, G. Demailly, M. Akkurt, S.Ö. Yýldýrým, T.B. Hadda, Arkivoc xvi, 19 (2007)
P. Ertl, B. Rohde, P. Selzer, J. Med. Chem. 43, 3714 (2000)
D.E. Clark, J. Pharm. Sci. 88, 807 (1999)
C.W. Chang, R.F. Spanjersberg, M.W. Beukers, A.P. IJzerman, J. Med. Chem. 47, 6529 (2004)
V.N. Viswanadhan, A.K. Ghose, G.R. Revankar, R.K. Robins, J. Chem. Inf. Comput. 29, 163 (1989)
C.A. Lipinski, F. Lombardo, B.W. Dominy, P.J. Feeney, Adv. Drug Deliv. Rev. 46, 3 (2001)
Acknowledgments
Professor Taibi Ben Hadda is indebted to the Celeron Company, Switzerland, for help with POM analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hadda, T.B., Fergoug, T. & Warad, I. POM theoretical calculations and experimental verification of the antibacterial potential of 5-hydroxy-4-(substituted-amino)-2(5H)-furanones. Res Chem Intermed 39, 1963–1971 (2013). https://doi.org/10.1007/s11164-012-0729-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0729-0