Abstract
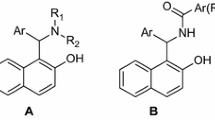
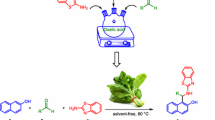
A series 1-amidoalkyl-2-naphthols were synthesized by one-pot three-component condensation reaction of β-naphthol with various aromatic aldehydes and amides under thermal (hot plate and oil bath) and microwave techniques. and the effect of various organoacids used as catalyst was studied. Among the various acids screened, p-aminobezene sulphonic acid (sulfanilic acid) came out to be a versatile catalyst and the corresponding products were obtained in good to excellent yield (84–94 %) under solvent-free condition.



Similar content being viewed by others
References
A.Y. Shen, C.T. Tsai, C.L. Chen, Eur. J. Med. Chem. 34, 877 (1999)
I. Szatmari, F. Fulop, Curr. Org. Synth. 1, 155 (2004)
M. Damodiran, N.P. Selvam, P.T. Perumal, Tetrahedron Lett. 50, 5474 (2009)
A.H. Kategaonkar, S.S. Sonar, K.F. Shelke, B.B. Shingate, M.S. Shingare, Org. Commun. 3, 1 (2010)
Y. Kusakabe, J. Nagatsu, M. Shibuya, O. Kawaguchi, C. Hirose, S. Shirato, J. Antibiot. 25, 44 (1972)
J.B. Chylińska, T. Urbański, M. Mordarski, J. Med. Chem. 6, 484 (1963)
J.L. Peglion, J. Vian, Bioorg. Med. Chem. Lett. 7, 881 (1997)
H. Ren, S. Grady, D. Gamenara, H. Heinzen, P. Moyna, S. Croft, H. Kendrick, V. Yardley, G. Moyna, Bioorg. Med. Chem. Lett. 11, 1851 (2011)
F. Benedini, G. Bertolini, R. Cereda, G. Doná, G. Gromo, S. Levi, J. Mizrahi, A. Sala, J. Med. Chem. 38, 130 (1995)
R.D. Clark, J.M. Caroon, A.F. Kluge, D.B. Repke, A.P. Roszkowski, A.M. Strosberg, S. Baker, S.M. Bitter, M.D. Okada, J. Med. Chem. 26, 657 (1983)
J.B. Chylińska, M. Janowiec, T. Urbański, Br. J. Pharmacol. 43, 649 (1971)
S. Knapp, Chem. Rev. 95, 1859 (1995)
S.M. Vrouenraets, W.F. Wit, J. Tongeren, J.M. Lange, Expert Opin. Phamacother. 8, 851 (2007)
M. Anary-Abbasinejad, A. Hassanabadi, M. Kamali-Gharamaleki, A. Saidipoor, H. Anaraki-Ardakani, J. Chem. Res. 644 (2007)
M.M. Khodaei, A.R. Khosropour, H. Moghanian, Synlett 916, 114 (2006)
H.R. Shaterian, H. Yarahmadi, ARKIVOC II, 105 (2008)
H.R. Shaterian, H. Yarahmadi, M. Ghashang, Bioorg. Med. Chem. Lett. 18, 788 (2008)
W.K. Su, W.Y. Tang, J.J. Li, J. Chem. Res. 123 (2008)
B. Das, K. Laxminarayana, B. Ravikanth, R. Rao, J. Mol. Catal. A: Chem. 261, 180 (2007)
A. Shahrisa, S. Esmati, M.G. Nazari, J. Chem. Sci. 124, 927 (2012)
S.B. Patil, P.R. Singh, M.P. Surpur, S.D. Samant, Synth. Commun. 37, 1659 (2007)
H.R. Shaterian, H. Yarahmadi, M. Ghashang, Tetrahedron 64, 1263 (2008)
B. Das, D.N. Kumar, K. Laxminarayana, B. Ravikanth, Helv. Chim. Acta 90, 1330 (2007)
H.R. Shateria, H. Yarahmadi, Tetrahedron Lett. 49, 1297 (2008)
S. Kantevari, S.V.N. Vuppalapati, L. Nagarapu, Catal. Commu. 8, 1857 (2007)
G. Srihari, M. Nagaraju, M.M. Murthy, Helv. Chim. Acta 90, 1497 (2007)
S.B. Patil, P.R. Singh, M.P. Surpur, S.D. Samant, Ultrason. Sonochem. 14, 515 (2007)
R.R. Nagawade, D.B. Shinde, Chin. J. Chem. 25, 1710 (2007)
G. Vaghei, S.M. Malaekehpour, Cent. Eur. J. Chem. 8, 1086 (2010)
A. Kumar, M.S. Rao, I. Ahmad, B. Khungar, Can. J. Chem. 87, 714 (2009)
H. Khabazzadeh, K. Saidi, N. Seyedi, J. Chem. Sci. 121, 429 (2009)
A.R. Hajipour, F. Ghayeb, N. Sheikhan, A.E. Ruoho, Tetrahedron Lett. 50, 5649 (2009)
S.B. Sapkal, K.F. Shelke, B.R. Madje, B.B. Shingate, M.S. Shingare, Bull. Korean Chem. Soc. 30, 2887 (2009)
T.T. She, Z.L. Liu, K. Gong, Chinese Journal Applied Chem. 27, 778 (2010)
P.T. Anastas, J.C. Warner, Green Chemistry: Theory and Practice (Oxford University Press, Oxford, 1998)
P.T. Anastas, T. Williamson, Green Chemistry, Frontiers in Benign Chemical Synthesis and Process (Oxford University Press, Oxford, 1998)
P.I.D. Peter, L. Moisan, Angew. Chem. Int. Ed. 43, 726 (2001)
R. Duvedi, R.K. Singh, Asian J. Chem. 24, 5665 (2012)
S. Sharma, D.N. Prasad, R.K. Singh, J. Chem. Pharm. Res. 3, 382 (2011)
A. Sandhar, D.N. Prasad, R.K. Singh, Ind. J. Het. Chem. 21, 369 (2012)
A. Sandhar, D.N. Prasad, A. Kapoor, R.K. Singh, Curr. Res. Chem. 4, 68 (2012)
S. Malik, S. Sharma, R.K. Singh, Asian J. Chem. 24, 5669 (2012)
P. Kaur, H. Sharma, R. Rana, D.N. Prasad, R.K. Singh, Asian J. Chem. 24, 5649 (2012)
A. Sandhar, R.K. Singh, Asian J. Chem. 24, 5643 (2012)
A. Sandhar, R.K. Singh, Chem. Sci. Trans. 2, 176 (2013)
P. Kaur, R.K. Singh, Chem. Sci. Trans. 2(S1), S295 (2013)
J.P. Patel, J.R. Avalani, D.K. Raval, J. Chem. Sci. 125, 531 (2013)
R.M. Borik, Aust. J. Basic Appl. Sci. 7, 543 (2013)
[49] U.P. Tarpada, B.B. Thummar, D.K. Raval, J. Saudi Chem. Soc., inpress 2012. doi.org/10.1016/j.jscs.2012.07.014
Acknowledgments
The authors gratefully acknowledged Dr. D.N. Prasad, Principal, and Management, Shivalik College of Pharmacy, Nangal, Punjab for constant encouragement and support. Thanks are also due to SAIF, Panjab University, Chandigarh for cooperation in getting the spectral data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, R.K., Singh, B., Duvedi, R. et al. Sulfanilic acid: a versatile and efficient catalyst among various organoacids screened for the synthesis of 1-amidoalkyl-2-naphthols under solvent-free condition. Res Chem Intermed 41, 4083–4099 (2015). https://doi.org/10.1007/s11164-013-1513-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1513-5