Abstract
The increasing clinical importance of drug-resistant fungal and bacterial pathogens has stimulated microbiological research into and development of new antimicrobial agents. Hence, in this study, we synthesized novel series of 2-(pyridin-3-yl)-1H-benzo[d]imidazoles and 2-(pyridin-3-yl)-3H-imidazo[4,5-b]pyridine derivatives. The structures of the compounds were confirmed by spectral and CHN analysis. The compounds were examined for in-vitro antimicrobial activity against Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis), Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa), and the fungus Candida albicans.
Graphical Abstract

Similar content being viewed by others
Introduction
In the last few decades, microbes have developed strong resistance to antimicrobial drugs [1, 2]. Development of this resistance has recently accelerated substantially, leading to an increase in the number of infections. As a result there is a constant need to develop antimicrobial drugs [3]. One major objective of organic and medicinal chemistry is to design and synthesize new molecules with high therapeutic indices which can overcome resistant microorganisms. Despite significant progress in antimicrobial therapy there is still much demand for novel antimicrobial drugs [4]. Because infectious disease [3] is a major global health problem [5], the resistance acquired by microbes may be because of increasing use and misuse of antimicrobial drugs [6, 7].
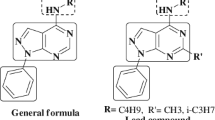
In this study we synthesized benzimidazole and imidazo[4,5-b]pyridine derivatives, because these structures are known to have a wide range of pharmacological activity [8–13]. Incorporation of an imidazole nucleus, a biologically well-established pharmacophore [13, 14], in 2-(pyridin-3-yl)-1H-benzo[d]imidazoles and 2-(pyridin-3-yl)-3H-imidazo[4,5-b]pyridine derivatives has resulted in versatile heterocyclic systems with a wide range of biological activity [13, 15–17].
Benzmidazole and imidazo[4,5-b]pyridine groups readily interact with the biopolymers of living organisms [18]. Because this type of structure is known to have a wide range of biological activity, for example antibacterial [19], antiviral [20], antimicrobial [21], antiulcer proton-pump inhibiting [22, 23], and anticancer [24] activity, and because, in the pharmaceutical sciences, new drugs are usually discovered on the basis of molecular modification of lead compounds or already established pharmacophores, we synthesized fourteen novel derivatives of 2-(pyridin-3-yl)-1H-benzo[d]imidazole and 2-(pyridin-3-yl)-3H-imidazo[4,5-b]pyridine.
Recent investigation of a variety of triazoles has revealed the importance of this pharmacophore to antibacterial activity [12, 13, 19]. Interestingly, the 1,2,3-triazole ring had been reported to mimic peptide bonds (amide bond surrogate) [25, 26]. 1,2,3-Triazoles can be regarded as antibacterial agents, because they can inhibit synthesis of the cell membrane, cell wall, and nucleic acids of bacteria [27]. We therefore synthesized 2-(pyridin-3-yl)-1H-benzo[d]imidazoles and 2-(pyridin-3-yl)-3H-imidazo[4,5-b]pyridine derivatives with or without methyl-1,2,3-triazole substitution (Scheme 1) and characterized the compounds by spectral and CHN analysis. The compounds were screened for antimicrobial activity, and minimum inhibitory concentration (MIC) was determined by use of the repeated twofold serial dilution technique (Table 4).
Result and discussion
A general multistep synthetic procedure used for preparation of 2-(pyridin-3-yl)-1H-benzo[d]imidazoles and 2-(pyridin-3-yl)-3H-imidazo[4,5-b]pyridine derivatives. A pyridine aldehyde (3a–c) was reacted with a benzene-1,2-diamine or pyridine-2,3-diamine (4a–f) in the presence of CAN/H2O2 to give 2-(pyridin-3-yl)-1H-benzo[d]imidazoles or 2-(pyridin-3-yl)-3H-imidazo[4,5-b]pyridine derivatives, respectively (5a–5k), in good yield and purity. The pyridine aldehyde intermediates (3a–c) were prepared directly or via enamide formation starting from pent-3-en-2-one oxime, 4-phenyl-3-butene-2-one-oxime, or methyl 2-acetamidoacrylate (Table 1) [28–31].
We then synthesized 1-((3H-1,2,3-triazol-4-yl)methyl)-2-(pyridin-3-yl)-1H-benzo[d]imidazole and 3-((3H-1,2,3-triazol-4-yl)methyl)-2-(pyridin-3-yl)-3H-imidazo[4,5-b]pyridine derivatives (6a–c) by C–N coupling of the N-alkylated methyltriazole compounds 2-(pyridin-3-yl)-1H-benzo[d]imidazoles and 2-(pyridin-3-yl)-3H-imidazo[4,5-b]pyridine derivatives (5a–k) in the presence of a base in DMF [32]. Details of the synthesis of all these new compounds are given in Scheme 1 and Table 2. All compounds were characterized by use of spectral techniques, for example 1H NMR, 13C NMR, and mass spectroscopy, and CHN analysis.
Biological evaluation
Because heterocyclic systems, especially benzimidazole and imidazo[4,5-b]pyridine derivatives, are known to be pharmacologically important [19–21], the novel 2-(pyridin-3-yl)-1H-benzo[d]imidazole and 2-(pyridin-3-yl)-3H-imidazo[4,5-b]pyridine compounds (5a–k) and their methyltriazole derivatives (6a–c) were screened for antibacterial and antifungal activity, in comparison with streptomycin and gentamicin, respectively, as reference standards. The results are summarized in Tables 3 and 4.
Antibacterial activity
Perusal of the results from antibacterial screening revealed that, compared with streptomycin, most of the compounds were effective against the Gram-positive bacteria Staphylococcus aureus (NCIM 5022) and Bacillus subtilis (NCIM 2545) and the Gram-negative bacteria Escherichia coli (NCIM 2036) and Pseudomonas aeruginosa (NCIM 2036). In particular, compounds 5b and 5c had promising activity, compounds 5d, 5e, 5g–k, and 6a–c had moderate activity, and compounds 5a and 5f had activity at high concentrations only. The MIC of all the compounds were also determined, by use of the broth-dilution method; the results are given in Table 4. Except for compounds 5a and 5f the MIC values were within the range 100–500 μg/ml−1 against all the strains evaluated.
Antifungal activity
The antifungal activity of the compounds (125 μg/ml) against Candida albicans (NCIM 3102), compared with gentamicin as standard, were evaluated by use of the cup plate method. The results obtained (Tables 3 and 4) revealed that compounds 5b and 5g had good antifungal activity whereas the activity of the other compounds was similar to or less than that of gentamicin.
Experimental
All reagents were purchased from Sigma–Aldrich, Lancaster, and Qualigens and were used without further purification. 1H NMR spectra were recorded on a Bruker Spectrospin Avance DPX400 Ultrashield (400 and 300-MHz) spectrometer, at room temperature; coupling constants, J, are in Hz. 13C NMR spectra were recorded on Bruker Spectrospin Avance DPX400 Ultrashield (100-MHz) spectrometer. Samples (2–3 mg) were dissolved in 0.8 ml deuterated CDCl3. Mass spectra were obtained by use of LC–MS with ESI for ionization. All reactions were monitored by thin layer chromatography (TLC) on silica gel with chloroform–acetone as mobile phase. The newly synthesized products were also separated and purified by column chromatography.
General procedure for synthesis of 2-(pyridin-3-yl)-1H-benzo[d]imidazoles and 2-(pyridin-3-yl)-3H-imidazo[4,5-b]pyridine derivatives

A mixture of benzene-1,2-diamine or pyridine-2,3-diamine (4a–f, 1 mmol), pyridine aldehyde (3a–c, 1 mmol), H2O2 (30 %, 4 mmol), and (NH4)2Ce(NO3)6 (CAN) (0.1 mmol) was heated to 50 ºC and maintained at this temperature for 2–4 h. Completion of the reactions was monitored by TLC (mobile phase chloroform–acetone 3:7; the starting materials and products are highly polar). On completion of the reaction, the mixture was dissolved in ethanol (10 ml) then poured into ice-cold water (30 ml). The solid product which precipitated was isolated by filtration, washed with ice-cold water, then dried under vacuum. The solvent was removed under reduced pressure to furnish off-white to yellow solids (5a–k, yield 82–91 %). The compounds were characterized by use of spectral techniques, for example 1H NMR, 13C NMR, and mass spectroscopy, and by CHN analysis.
2-(2-Chloro-5-phenylpyridin-3-yl)-1H-benzo[d]imidazole (5a)
Yellow solid, 1H NMR (400 MHz, CDCl3, ppm): δ 8.69–8.62 (2H, m), 8.41–8.36 (1H, m), 8.06–8.04 (1H, d, J = 7.6 Hz), 7.62–7.60 (2H, d, J = 8.0 Hz), 7.53–7.51 (1H, d, J = 8.0 Hz), 7.47–7.37 (4H, m), 7.23–7.21 (1H, m). 13C NMR (100 MHz, CDCl3, ppm): δ 166.62, 149.41, 148.58, 146.88, 144.98, 139.10, 138.75, 136.21, 135.50, 129.26, 128.88, 127.11, 127.00, 125.87, 118.79. ESI–MS: m/z 307.0, C18H12ClN3 requires Mol. Wt.: 305.76. Elemental analysis, calculated: C, 70.71; H, 3.96; Cl, 11.60; N, 13.74 %. Found: C, 70.75; H, 3.98; N, 13.72 %.
2-(2-Chloro-5-methylpyridin-3-yl)-5-methyl-1H-benzo[d]imidazole (5b)
Off white solid, 1H NMR (300 MHz, CDCl3, ppm): δ 8.28 (1H, s), 8.11 (1H, s), 7.51 (1H, bs), 7.07–7.05 (2H, d, J = 6.9 Hz), 2.63 (3H, s), 2.43 (3H, s). 13C NMR (100 MHz, CDCl3, ppm): δ 169.31, 166.08, 164.81, 160.74, 152.28, 145.26, 142.72, 142.49, 131.61, 115.20, 35.84, 36.45. ESI–MS; m/z; 258.00, C14H12ClN3 requires Mol. Wt.: 257.72. Elemental analysis, calculated: C, 65.25; H, 4.69; Cl, 13.76; N, 16.30 %. Found: C, 65.70; H, 4.81; N, 16.74 %.
2-(2-Chloro-5-phenylpyridin-3-yl)-5-methyl-1H-benzo[d]imidazole (5c)
Yellow solid, 1H NMR (300 MHz, CDCl3, ppm): δ 8.68–8.67 (2H, d, J = 2.1 Hz), 7.70–7.68 (2H, d, J = 7.2 Hz), 7.52–7.41 (4H, m), 7.21–7.16 (1H, d, J = 7.5 Hz), 7.08–7.06 (1H, d, J = 7.5 Hz), 2.68 (3H, s). 13C NMR (75 MHz, CDCl3, ppm): δ 167.00, 165.92, 158.35, 155.39, 154.50, 148.40, 148.01, 146.14, 145.56, 142.80, 142.66, 35.92. ESI–MS: m/z 320.00, C19H14ClN3 requires Mol. Wt.: 319.79. Elemental analysis, calculated: C, 71.36; H, 4.41; Cl, 11.09; N, 13.14 %. Found: C, 71.77; H, 4.61; N, 13.54 %.
Methyl 6-chloro-5-(5-methyl-1H-benzo[d]imidazol-2-yl)picolinate (5d)
Light yellow solid, 1H NMR (400 MHz, CDCl3, ppm): δ 9.05–9.03 (1H, d, J = 8.0 Hz), 8.22–8.20 (1H, d, J = 8.0 Hz), 7.54–7.53 (1H, d, J = 7.6 Hz), 7.29–7.26 (1H, t, J = 7.2 Hz), 7.17–7.15 (1H, d, J = 7.2 Hz), 4.05 (3H, s), 2.69 (3H, s). 13C NMR (100 MHz, CDCl3, ppm): δ 163.97, 147.73, 146.55, 144.86, 141.82, 128.76, 124.32, 53.38, 29.71. ESI–MS: m/z 302.00, C15H12ClN3O2 requires Mol. Wt.: 301.73. Elemental analysis, calculated: C, 59.71; H, 4.01; Cl, 11.75; N, 13.93; O, 10.61 %. Found: C, 59.64; H, 4.86; N, 13.48 %.
5-Bromo-2-(2-chloro-5-methylpyridin-3-yl)-3H-imidazo[4,5-b]pyridine (5e)
Yellow solid, 1H NMR (300 MHz, CDCl3, ppm): δ 8.87 (1H, s), 8.30 (1H, s), 7.95 (1H, bs), 7.44–7.47–7.44 (1H, m), 2.44 (3H, s). 13C NMR (100 MHz, CDCl3, ppm): δ 147.53, 146.55, 144.86, 141.76, 128.08, 124.36, 29.76. Mass: ESI–MS: m/z 325.00 (M+H)+, C12H8BrClN4 requires Mol. Wt.: 323.58. Elemental analysis, calculated: C, 44.54; H, 2.49; Br, 24.69; Cl, 10.96; N, 17.31. Found: C, 44.46; H, 3.69; N, 17.29.
(2-(2-Chloro-5-methylpyridin-3-yl)-1H-benzo[d]imidazol-5-yl)(phenyl)methanone (5f)
Yellow solid, 1H NMR (400 MHz, CDCl3, ppm): δ 11.38 (1H, bs, NH), 8.48 (1H, bs), 8.25 (1H, bs), 8.15 (1H, bs), 7.84–7.78 (3H, m), 7.73–7.68 (1H, bs), 7.58–7.54 (1H, t, J = 7.2 Hz), 7.47–7.43 (2H, t, J = 7.6 Hz), 2.35 (3H, s). 13C NMR (100 MHz, CDCl3, ppm): δ 196.88, 150.99, 144.76, 141.26, 138.01, 133.38, 132.27, 130.96, 130.02, 128.27, 125.70, 124.48, 17.53. ESI–MS: m/z 348.0, C20H14ClN3O requires Mol. Wt.: 347.80. Elemental analysis, calculated: C, 69.07; H, 4.06; Cl, 10.19; N, 12.08; O, 4.60 %. Found: C, 69.02; H, 4.16; N, 10.19 %.
(2-(2-Chloro-5-phenylpyridin-3-yl)-1H-benzo[d]imidazol-5-yl)(phenyl)methanone (5g)
Light yellow solid, 1H NMR (400 MHz, CDCl3, ppm): δ 9.12 (1H, s), 8.72 (1H, s), 8.23 (1H, s), 7.94–7.92 (1H, d, J = 8.0 Hz), 7.86–7.84 (3H, d, J = 8.4 Hz), 7.71–7.69 (2H, d, J = 6.8 Hz), 7.66–7.60 (1H, m), 7.55–7.44 (5H, m). 13C NMR (100 MHz, CDCl3, ppm): δ 196.35, 145.76, 139.17, 137.91, 136.73, 135.11, 133.67, 132.38, 130.06, 129.34, 129.11, 128.36, 127.23, 126.31. ESI–MS: m/z 410.00, C25H16ClN3O requires Mol. Wt.: 409.87. Elemental analysis, calculated: C, 73.26; H, 3.93; Cl, 8.65; N, 10.25; O, 3.90 %. Found: C, 73.11; H, 4.10; N, 10.33 %.
2-(2-Chloro-5-phenylpyridin-3-yl)-3H-imidazo[4,5-b]pyridine (5h)
Yellow solid, 1H NMR (400 MHz, CDCl3, ppm): δ 8.69–8.62 (2H, m), 8.41–8.36 (1H, m), 8.06–8.04 (1H, d, J = 7.6 Hz), 7.62–7.60 (2H, d, J = 8.0 Hz), 7.53–7.51 (1H, d, J = 8.0 Hz), 7.47–7.37 (3H, m). 13C NMR (100 MHz, CDCl3, ppm): δ 149.41, 148.58, 146.88, 144.98, 139.10, 138.75, 136.21, 135.50, 129.26, 128.88, 127.11, 127.00, 125.87, 118.79. LC–MS: m/z 307.0, C17H11ClN4 requires Mol. Wt.: 306.75. Elemental analysis, calculated: C, 66.56; H, 3.61; Cl, 11.56; N, 18.26 %. Found: C, 66.33; H, 3.97; N, 18.52 %.
2-(2-Chloro-5-phenylpyridin-3-yl)-5-nitro-1H-benzo[d]imidazole (5i)
Yellow solid, 1H NMR (300 MHz, CDCl3, ppm): δ 8.75–8.56 (3H, m), 8.25 (1H, bs), 7.72–7.69 (2H, d, J = 7.2 Hz), 7.55–7.47 (4H, m). 13C NMR (100 MHz, CDCl3, ppm): δ 148.41, 139.05, 136.20, 129.06, 128.76, 126.71, 118.98, 115.57, 111.76, 95.81. ESI–MS: m/z 351.00, C18H11ClN4O2 requires Mol. Wt.: 350.76. Elemental analysis, calculated: C, 61.64; H, 3.16; Cl, 10.11; N, 15.97;O, 9.12 %. Found: C, 61.49; H, 3.48; N, 15.77 %.
Methyl 6-chloro-5-(5-nitro-1H-benzo[d]imidazol-2-yl)picolinate (5j)
Yellow solid, 1H NMR (400 MHz, CDCl3, ppm): δ 9.05–9.03 (1H, d, J = 8.0 Hz), 8.22–8.20 (1H, d, J = 8.0 Hz), 7.54–7.53 (1H, d, J = 7.6 Hz), 7.29–7.26 (1H, t, J = 7.2 Hz), 7.17–7.15 (1H, d, J = 7.2 Hz), 4.05 (3H, s). 13C NMR (100 MHz, CDCl3, ppm): δ 163.97, 147.73, 146.55, 144.86, 141.82, 128.76, 124.32, 114.22, 53.38. LC–MS: m/z 333.50, C14H9ClN4O4 requires Mol. Wt.: 332.70. Elemental analysis, calculated: C, 50.54; H, 2.73; Cl, 10.66; N, 16.84; O, 19.24. Found: C, 50.38; H, 3.18; N, 16.72 %.
Methyl 5-(1H-benzo[d]imidazol-2-yl)-6-chloropicolinate (5k)
Yellow solid, 1H NMR (400 MHz, CDCl3, ppm): δ 8.90–8.88 (1H, d, J = 8.0 Hz), 8.18–8.16 (1H, d, J = 8.0 Hz), 7.72–7.69 (2H, m), 7.36–7.34 (2H, m), 4.02 (3H, s). 13C NMR (100 MHz, CDCl3, ppm): δ 167.89, 163.91, 147.83, 147.01, 145.58, 141.80, 138.40, 132.38, 130.94, 128.79, 124.24, 115.83, 53.37. LC–MS: m/z 287.90, C14H10ClN3O2 requires Mol. Wt.: 287.70. Elemental analysis, calculated: C, 58.45; H, 3.50; Cl, 12.32; N, 14.61; O, 11.12 %. Found: C, 58.30; H, 3.81; N, 14.58 %.
General procedure for alkylation of 2-(pyridin-3-yl)-1H-benzo[d]imidazoles and 2-(pyridin-3-yl)-3H-imidazo[4,5-b]pyridine derivatives

A suspension of 60 % sodium hydride (2.0 equiv.) and dry N,N-dimethylformamide (eightfold volume excess) was stirred for 5–10 min under a nitrogen atmosphere. A solution of compound 5a–k (1 mmol) in dry N,N-dimethylformamide (fivefold volume excess) was slowly added, and stirring was continued for an additional 30–60 min. A solution of 5-(bromomethyl)-1H-1,2,3-triazole (1.1 equiv.) in N,N-dimethylformamide (fivefold volume excess) was then added dropwise, and the mixture was left overnight at room temperature. Completion of the reactions (10–14 h) was monitored by TLC (mobile phase chloroform–acetone 3:7; the starting materials and products are highly polar). On completion, the reaction mixture was quenched with saturated ammonium chloride solution, concentrated under reduced pressure to remove volatile materials, then extracted with ethyl acetate. The organic extract was filtered through Celite and washed with water then saturated brine solution. The organic layer was dried over anhydrous Na2SO4, then the solvent was removed by evaporation under reduced pressure to give 6a–c as yellow solids (yield: 80–90 %). The compounds were characterized by use of spectral techniques, for example 1H NMR, 13C NMR, and mass spectroscopy, and by CHN analysis.
1-((3H-1,2,3-Triazol-4-yl)methyl)-2-(2-chloro-5-methylpyridin-3-yl)-5-methyl-1H-benzo[d] imidazole (6a)
Yellow solid, 1H NMR (400 MHz, CDCl3, ppm): δ 11.84 (1H, bs), 9.11 (1H, s), 8.84–8.72 (1H, m), 8.33 (1H, bs), 7.82–7.81 (1H, bs), 7.72–7.71 (2H, d, J = 6.0 Hz), 4.11 (2H, s), 2.42 (3H, s), 2.32 (3H, s). 13C NMR (100 MHz, CDCl3, ppm): δ 157.37, 149.83, 149.22, 147.73, 146.55, 144.86, 141.82, 134.21, 133.27, 131.55, 128.61, 124.42, 122.82, 116.75, 49.28, 22.70, 20.05. ESI–MS: m/z 339.90, C17H15ClN6 requires Mol. Wt.: 338.79. Elemental analysis, calculated: C, 60.27; H, 4.46; Cl, 10.46; N, 24.81 %. Found: C, 60.54; H, 4.96; N, 24.90 %.
Methyl 5-(1-((3H-1,2,3-triazol-4-yl)methyl)-5-methyl-1H-benzo[d]imidazol-2-yl)-6-chloro pyridine-2-carboxylate (6b)
Yellow solid, 1H NMR (400 MHz, CDCl3, ppm): δ 11.84 (1H, bs), 9.11 (1H, s), 8.84–8.72 (1H, m), 8.33–8.31 (1H, bs), 7.82–7.81 (1H, bs), 7.72–7.71 (2H, d, J = 6.0 Hz), 4.12 (2H, s), 3.94 (3H, s), 2.42 (3H, s). 13C NMR (100 MHz, CDCl3, ppm): δ 163.97, 157.27, 149.23, 147.73, 146.55, 144.86, 141.82, 134.17, 133.27, 131.55, 128.76, 124.32, 122.82, 116.85, 53.38, 22.70. ESI–MS: m/z 383.00, C18H15ClN6O2requires Mol. Wt.: 382.80. Elemental analysis, calculated: C, 56.48; H, 3.95; Cl, 9.26; N, 21.95; O, 8.36 %. Found: C, 56.82; H, 4.46; N, 21.98 %.
3-((3H-1,2,3-Triazol-4-yl)methyl)-2-(2-chloro-5-phenylpyridin-3-yl)-3H-imidazo[4,5-b]pyridine (6c)
Yellow solid, 1H NMR (400 MHz, CDCl3, ppm): δ 11.71 (1H, bs), 8.69–8.62 (2H, m), 8.41–8.36 (1H, m), 8.06–8.04 (1H, d, J = 7.6 Hz), 7.47–7.32 (5H, m), 7.72–7.71 (2H, d, J = 6.0 Hz), 3.72 (2H, s). 13C NMR (100 MHz, CDCl3, ppm): δ 151.62, 149.41, 148.84, 148.58, 146.88, 144.98, 139.10, 138.75, 136.21, 135.50, 135.42, 129.26, 128.88, 128.77, 127.46, 127.11, 127.00, 125.88, 118.79, 50.95. ESI–MS: m/z 388.00, C20H14ClN7 requires Mol. Wt.:387.83. Elemental analysis, calculated: C, 61.94; H, 3.64; Cl, 9.14; N, 25.28 %. Found: C, 62.68; H, 4.18; N, 25.18 %.
Minimum inhibitory concentration (MIC)
Sterile nutrient agar (Sabourd dextrose agar) plates were prepared by pouring the sterile agar into sterile Petri dishes under aseptic conditions and the test organism (0.1 ml) was spread on the plates. Holes (5 mm diameter) were made in the agar plates by use of a sterile bore. Test compound, standard drug, and the DMSO (as control) were placed in separate holes. The plates were maintained at +4 °C for 1 h to enable diffusion of the solutions into the agar medium. Plates containing bacteria were incubated at 37 °C for 24 h; those containing fungi were incubated at 28 °C for 48 h.
Determination of MIC in liquid medium
A series of test tubes were prepared containing the same volume of medium inoculated with the test organism (the inoculum may vary from 103 to 106 cells per milliliter). Decreasing concentrations of the test compounds were added to the tubes; stepwise dilution by a factor of 2 (twofold serial dilution) was usually used (500 μg/ml, 250 μg/ml, 125 μg/ml, etc.) [33, 34]. One tube was left without test compound, to serve as a positive control for growth of the organism. The cultures were incubated. The tubes were inspected visually to monitor growth of the organism (indicated by turbidity);tubes containing the antimicrobial agent at a concentration sufficient to inhibit growth remained clear. Experimentally, the MIC is the concentration of the test compound present in the last clear tube, i.e. the tube containing the lowest concentration of test compound in which growth is not observed.
The synthesized compounds were checked, in vitro, for inhibitory activity against five microorganisms—the bacteria S. aureus, B. subtilis, E. coli, and P. aeruginosa and the fungus C. albicans. Streptomycin (125 μg/ml) and gentamicin (125 μg/ml) were used as controls.
Conclusion
In this study we synthesized 2-(pyridin-3-yl)-1H-benzo[d]imidazoles and 2-(pyridin-3-yl)-3H-imidazo[4,5-b]pyridine derivatives by use of a CAN/H2O2 catalytic system and characterized the compounds by spectral and CHN analysis. We also synthesized N-alkylated 1,2,3-triazolemethyl derivatives. All the compounds were screened for antibacterial and antifungal activity, and found to have moderate to good activity.
References
Ion Chopra, J. Antimicrob. Chemother. 30, 737 (1992)
S. Zhou, F. Li, P. Zhang, L. Jiang, Res. Chem. Intermed. 39, 1735 (2013)
O. A. Phillipsa, E. E. Udo, M. E. Abdel-Hamida, R. Varghesea, Eur. J. Med. Chem. 44, 3217 (2009)
J.P. Donnelly, A. Voss, W. Witte, B.E. Murray, J. Antimicrob. Chemother. 37, 389 (1996)
M. Venkatesh, V.G. Bairavi, K.C. Sasikuma, J. Pharm. Bio allied Sci. 3, 101 (2011)
P. Little, C. Gould, I. Williamson, G. Warner, M. Gantley, A.L. Kinmonth, BMJ 315, 350 (1997)
W. Wilson, K.A. Taubert, M. Gewitz, Circulation 116, 1736 (2007)
L. Wang, H. Ankati, S. K. Akubathini, M. Balderamos, C. A. Storey, A. V. Patel, V. Price, D. Kretzschmar, E. R. Biehl, S. R. D’Mello, J. Neurosci. Res. 88, 1970 (2010)
D. I. Andersson, D. Hughes, Nat. Rev. Microbiol. 8, 260 (2010)
E. Nicolaï, J. Goyard, T. Benchetrit, J.M. Teulon, F. Caussade, A. Virone, C. Delchambre, A. Cloarec, J. Med. Chem. 36, 1175 (1993)
J. A. Kumar, A. K. Tiwari, A. Z. Ali, K. Madhusudhana, B. S. Reddy, S. Ramakrishna, B. China Raju, J. Enzyme Inhib. Med. Chem. 25, 80 (2010)
A. K. Jordão, P. C. Sathler, V. F. Ferreira, V. R. Campos, M. C. de Souza, H. C. Castro, A. Lannes, A. Lourenco, C. R. Rodrigues, M. L. Bello, M. C. Lourenco, G. S. Carvalho, M. C. Almeida, A. C. Cunha, Bioorg. Med. Chem. 19, 5605 (2011)
C. Gill, G. Jadhav, M. Shaikh, R. Kale, A. Ghawalkar, D. Nagargoje, M. Shiradkar, Bioorg. Med. Chem. Lett. 18, 6244 (2008)
B. Narasimhan, D. Sharma, P. Kumar, Med. Chem. Res. 20, 1119 (2011)
U. Velaparthi, M. Wittman, P. Liu, K. Stoffan, K. Zimmermann, X. Sang, J. Carboni, A. Li, R. Attar, M. Gottardis, A. Greer, C. Y. Chang, B. L. Jacobsen, J. S. Sack, Y. Sun, D. R. Langley, Bioorg. Med. Chem. Lett. 17, 2317 (2007)
A. Puratchikody, G. Nagalakshmi, M. Doble, Chem. Pharm. Bull. (Tokyo). 56, 273 (2008)
A.R. Katritzky, G. Qiu, Q.H. Long, H.Y. He, P.J. Steel, J. Org. Chem. 65, 9201 (2000)
M. Kimoto, T. Mitsui, Y. Harada, A. Sato, S. Yokoyama, I. Hirao, Nucleic Acids Res. 35, 5360 (2007)
F. Reck, F. Zhou, M. Girardot, G. Kern, C.J. Eyermann, N.J. Hales, R.R. Ramsay, M.B. Gravestock, J. Med. Chem. 48, 499 (2005)
M. Tonelli, M. Simone, B. Tasso, F. Novelli, V. Boido, F. Sparatore, G. Paglietti, S. Pricl, G. Giliberti, S. Blois, C. Ibba, G. Sanna, R. Loddo, P. La Colla, Bioorg. Med. Chem. 18, 2937 (2010)
A. H. El-masry, H. H. Fahmy, S. H. Ali Abdelwahed, Molecules 5, 1429 (2000)
J. M. Shin, K. Munson, O. Vagin, G. Sachs, Eur. J. Physiol. 457, 609 (2008)
J. M. Shin, M. Homerin, F. Domagala, H. Ficheux, G. Sachs, Biochem. Pharmacol. 71, 837 (2006)
Z. M. Nofal, E. A. Soliman, S. S. Abd El-Karim, M. I. El Zahar, A. M. Srour, S. Sethumadhavan, T. J. Maher, Acta Pol. Pharm. 68, 519 (2011)
A. Brik, J. Alexandratos, Y.-C. Lin, J. H. Elder, A. J. Olson, A. Wlodawer, D. S. Goodsell, C.-H. Wong, Chem. BioChem. 6, 1167 (2005)
M. Kume, T. Kubota, Y. Kimura, K. Nakashimizu, M. Motokawa, M.J. Nakano, Antibiotics 46, 177 (1993)
J. L. Hartwell, B. J. Abbot, Advances in Pharmacology and Chemotherapy, 7th edn. (Academic Press, New York, 1969), p. 56
R.R. Amaresh, P.T. Perumal, Synth. Commun. 30, 2269 (2000)
S. Ahmed, R. C. Boruah, Tetrahedron Lett. 37, 8231 (1996)
N. Garton, N. Bailey, M. Bamford, E. Demont, I. Farre-Gutierrez, G. Hutley, G. Bravi, P. Pickering, Bioorg. Med. Chem. Lett. 20, 1049 (2010)
B. Gangadasu, P. Narender, S. Bharath Kumar, M. Ravinder, B. AnandaRao, Ch. Ramesh, B. China Raju, V. Jayathirtha Rao, Tetrahedron 62, 8398 (2006)
Benson et al. US Pat. Appl. Publ. US20110077273 (2011)
A. Jarrahpour, D. Khalili, E. De Clercq, C. Salmi, J.M. Brunel, Molecules 12, 1720 (2007)
Indian Pharmacopoeia (Ministry of Health and Family Welfare New Delhi) A-114 (1996)
Acknowledgments
The authors wish to express their gratitude to the management, JNT University, for their support and facilities.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Mallemula, V.R., Sanghai, N.N., Himabindu, V. et al. Synthesis and characterization of antibacterial 2-(pyridin-3-yl)-1H-benzo[d]imidazoles and 2-(pyridin-3-yl)-3H-imidazo[4,5-b]pyridine derivatives. Res Chem Intermed 41, 2125–2138 (2015). https://doi.org/10.1007/s11164-013-1335-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1335-5