Abstract
The majority of osteoporotic fractures happen in individuals with BMD t-scores in the osteopenic range (−2, 5< t-score <−1). However, widespread use of anti-osteoporotic medication in this group based on t-score alone is not advisable because: 1) the number needed to treat is much higher (NNT > 100) than in patients with fractured and t-score below −2,5 (NNT 10–20); 2)while specific osteoporosis treatments have demonstrated significant reductions of the fracture risk in patients with t-score <−2, 5, the efficacy in patients in the osteopenic range is less well established. Therefore, an osteopenic t-score does not in itself constitute a treatment imperative. Generally, osteopenia has to be associated with either low energy fracture(s) or very high risk for future fracture as assessed with risk calculators like FRAX to warrant specific osteoporosis therapy. Vertebral fractures are now conveniently assessed using lateral x-rays from DXA machines. In the vast majority of cases antiresorptive treatments (mainly hormone replacement therapy and SERMS in younger and bisphosphonates or Denosumab in older women) are the treatments of choice in this group of patients,—only rarely is anabolic therapy indicated.
Similar content being viewed by others
1 Introduction
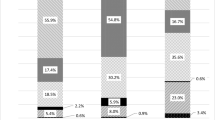
While pharmacological treatment of patients with prevalent osteoporotic fractures is universally accepted, the treatment of patients at increased risk of fracture due to low bone mass is more controversial. Our ability to detect patients at increased risk has improved significantly after the widespread availability of Dual Energy X-ray Absorptiometry (DXA), which provides a precise assessment of the amount of mineralized bone in the skeleton. According to the WHO criteria for assessment of DXA measurements, patients are considered having low bone mass (osteopenic), when their BMD t-score of the spine or hip lies between −1 and −2,5. Although fracture risk increases with decreases in BMD, the vast majority of osteoporotic fractures occur in osteopenic patients. This is due to the fact that even though he risk of fracture is lower in the osteopenia than in osteoporosis, the number of subjects at risk is much higher in the osteopenic range due to the Gaussian distribution of BMD values in the population (Fig. 1). In an analysis of self reported fractures from the National Osteoporosis Risk Assessment (NORA) study Siris et al. [1] reported that 82% of postmenopausal women with fractures had T scores better than −2.5. The study comprised 149 524 white postmenopausal women aged 50 to 104 years (mean age, 64.5 years). New fractures were reported by 2,259 women, including 393 hip fractures; but only 6.4% exhibited baseline T scores of −2.5 or less. Although fracture rates were highest in women with a t-score <−2,5, only 18% of the osteoporotic fractures and 26% of hip fractures occurred in this group.
Distribution of fracture rates and number of women with fractures according to BMD t-scores from the The National Osteoporosis Risk Assessment (NORA) study, (NORA), which comprised 149,524 white postmenopausal women aged 50 to 104 years (mean age, 64.5 years). Bone mineral density (BMD) was assessed by peripheral bone densitometry at the heel, finger, or forearm. Although fracture rates were highest in women with the lowest t-scores (open bars), the largest absolute number of fractures (black bars) was seen in the osteopenic range of t-score (−1 to −2,5). From ref.1 with permission
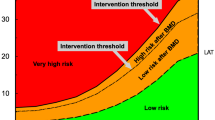
Osteoporosis leading to low energy fracture is often compared to hypertension leading to stroke. We have implemented widespread screening for high blood pressure and the intervention threshold is low. Thus, from a general health point of view, it would be beneficial to detect individuals at risk for low energy fracture and intervene before the catastrophic event,—with all its potential complications, happened. However, most clinicians are still hesitant implying aggressive pharmacologic treatment strategies in osteopenic patients. Several reasons for this apprehension have been cited:
First, perceptions around disease severity are different when comparing hypertension and osteoporosis. in the mind of most clinicians. Osteoporotic fracture is considered a far less severe event than stroke, although the increase in mortality and morbidity after spine or hip fractures is significant [2, 3].
Second, pharmaco-economic considerations also play a role. A lot of these discussions have centered around estimates of numbers needed to treat (NNT). While the most effective antiresorptive treatment today have NNT values for prevention of one fracture around 13–15 for spine fractures similar numbers in osteopenia patients are 8–10 fold higher [4, 5]. As shown in the analysis of Quandt et al. [4] the presence of a prevalent fracture yields a NNT for subsequent fracture over the next 5 years of 26, compared to 125 in patients without fractures at entry into the study. The fact that most patients with osteopenia are younger than the typical osteoporotic women is also important. Riancho (1999) reported that NNT for hip fractures ranged from 7 (for women aged 80 with a bone mineral density [BMD] with Z-scores <−2) to 333 (for women aged 50 with Z-scores between 0 and −1). Based on these assumptions they calculated that therapy of 100 women aged 80 years with a Z value <−2 for 3 years would prevent 14 hip fractures, 33 vertebral fractures and 8 wrist fractures. Therapy of 100 women aged 50–55 years with a Z value ranging from 0 to −1 would only prevent 1 hip fracture, 2 vertebral fractures and 2 wrist fractures [6]. Along the same lines Lamy and Krieg reported the results of economic analyses, which took into account the health care resources needed to get these benefits. They found that at 50 years, only calcium and vitamin D was cost-effective, whereas at 70 years, bisphosphonates and raloxifene were also cost-effective [7].
Third, a few studies, in particular on bisphosphonates, have hinted that clinical fractures were only significantly reduced in patients with t-scores <−2,5 [8]. In the same study, however, morphometric fractures were reduced even in patients with a t-score >2,5.
Most guidelines for osteopenic patients therefore primarily emphasize lifestyle changes like smoking cessation, nutritional improvements, calcium and vitamin D supplementation, exercise regimens etc. as primary interventions [9]. However, as seen from the NORA data, a significant number of high-risk osteopenic patients will still need pharmacologic intervention in order to reduce their fracture risk significantly. Identification of these individuals, however, demand effective case finding strategies. This review will summarize the current status of such strategies and the treatment options available and end op proposing a treatment algorithm based on this information.
1.1 Case finding strategies
While bone density remains one of the most valid and reliable measures of fracture risk, a better delineation of risk factors has led to renewed interest in absolute risk (AR) models such as FRAX. New imaging approaches, including lateral VFA, have been added to the diagnostic armamentarium, and facilitate identification of fractures earlier and with less radiation exposure to the patient. This is important because of the severe consequences of prevalent fractures in osteopenia as well as osteoporosis, not only of the hip but also of the much more common spine fractures.
1.1.1 Identification of osteopenic patients at increased risk of fracture
BMD is related to bone strength and low BMD is a major risk factor for fractures. However, most patients presenting with a fracture do not have BMD based osteoporosis, defined according to the World Health Organization (WHO) definition as a T score of −2.5 or below. The most poignant example is hip fracture, where only half the patients exhibit t-scores below −2.5 [10]. In addition, and independent of bone-related risks, extraskeletal risk factors such as falls contribute to fracture risk and are present in the majority of patients older than 50 years presenting with a clinical fracture, and falls are the dominant event leading to forearm and hip fracture [11].
1.1.2 Identification of patients with prevalent fractures
The primary risk factor for subsequent fracture is a prevalent low energy fracture, irrespective of whether it is clinically apparent or not. Thus, most guidelines for treatment consider the presence of a low energy fracture in an osteopenic patient a clear indication for specific osteoporosis therapy [9, 12, 13]. A history of nonvertebral fracture is associated with a doubling of the risk of a subsequent fracture, and the subsequent fracture risk is even quadrupled after a vertebral fracture. The re-fracture risk is, however, not constant over time. It is highest (2–3X) in the years immediately after a first fracture, followed by a gradual waning later on [14]. Forty to 50% of all subsequent fractures occur within 3 to 5 years after a first fracture, and the presence of such fractures demands rapid intervention with specific osteoporosis drugs to reduce the risk of a subsequent fracture. Prevalent hip, spine and several other nonvertebral fractures are all associated with increased morbidity and mortality [15], which is higher immediately after fracture than later on. Hip, vertebral, and nonhip, nonvertebral fractures were each associated with approximately one third of deaths. The major causes of death were related to cardiovascular and respiratory comorbidities [15].
Unfortunately subsequent follow up of fracture patients after orthopedic fracture repair to identify patients in need of specific osteoporosis treatment is still very limited. Most studies show that only 10–15% of fracture patients treated at orthopedic departments are offered a DXA evaluation, and even less patients ar offered supplementation with vitamin D and Ca or specific osteoporosis treatment. Fortunately a lot of centres are recognizing this dilemma and have established initiatives for post-fracture care (e.g. fracture liaison service [16]. Such interventions have the potential to reduce subsequent fractures, morbidity, mortality and readmissions to hospital.
While hip and other nonvertebral fractures are clinically obvious, the detection of vertebral fractures constitutes a significant problem. Morphometric vertebral fractures are the most frequent fractures in women and men older than 50 years [17] and their presence is a strong predictor of future vertebral, nonvertebral, and hip fracture risk [18, 19]. Clinical vertebral fractures are characterized by back pain lasting for 2–3 months, depending on fracture severity, but they represent only a small subgroup of all vertebral fractures. In recent large scale trials they constitute less than 10% of all morphometric fractures [20, 21]. Most morphometric vertebral fractures therefore remain undiagnosed, which results in many patients developing severe osteoporosis with multiple fractures and chronic pain, before effective treatment is instituted. Only when clinical suspicion, e.g. significant height loss, increasing kyphosis, protruding abdomen, rib-iliac crest distance of less than 2 cm, and acute or chronic back pain, is raised, a spine x-ray is performed. But even when lateral x-rays of the spine are available, vertebral fractures are often missed [22, 23].
Thus, detection of prevalent fractures is very important when making decisions on treatment in osteopenic women. This has been further facilitated by accessory software for DXA scanners yielding lateral x-rays of the spine, which permit assessment of vertebral fracture status. This procedure has been given many names: (vertebral morphometry, lateral vertebral assessment (LVA), vertebral fracture assessment (VFA) (Fig. 2). The images are usually of good quality, albeit less detailed than conventional x-rays, and in most cases a good evaluation of compression fractures in the range Th4-L4 is possible. Advantages are: low radiation dose, the availability of semiautomatic image analysis tools to assist in measuring vertebral shapes of the individual vertebrae, its plan-parallel projection, and its high negative predictive value. The disadvantage is the inability to study upper thoracic vertebrae, but only a minority of fractures are found there. Indications for VFA according to the ISCD [22] are shown in Table 1.
If pathology outside this region of interest (ROI) is suspected, other imaging techniques will have to used. The experience in most centres employing this methodology is, however, that such referrals are needed in less than 10% of cases. According to the International Society of Clinical Densitometry (ISCD), additional x-ray imaging is needed in cases of two or more mild (grade 1) deformities without any moderate or severe (grade 2 or 3) deformities, when lesions in vertebrae cannot be ascribed to benign causes, or when vertebral deformities are found in a patient with a known history of a relevant malignancy [22]. The methodology also permits assessment of spondylosis and even arteriosclerosis of the abdominal aorta can be evaluated.
The prevalence of previously unknown morphometric vertebral fractures has been studied in various at-risk populations. In a recent study of women and men presenting with a nonvertebral fracture, one out of four had a prevalent morphometric vertebral fracture on VFA that was not recognized previously [24]. In another study, the prevalence of morphometric vertebral fractures was 21% in postmenopausal women with osteopenia [25].
In patients with BMD-diagnosed osteoporosis, a baseline VFA is not necessary for treatment decisions but is helpful in detecting lack of treatment efficacy during follow-up. Fractures occurring in L1-L4 will increase apparent BMD, and may be difficult to see on the standard AP image provided by a routine scan.
1.1.3 Identification of high risk individuals without fracture history - the fracture risk assessment tool (FRAX)
As mentioned in the introduction, the vast majority of osteoporotic fractures take place in osteopenic patients without prevalent fractures. Many aspects of osteoporosis and fracture risk are clinically recognizable (such as age, gender, and body weight), even before a first fracture has occurred. Relative risk estimates are, however, difficult to apply in daily clinical practice since their clinical significance depends on the prevalence of fractures in the general population. In order to better delineate individuals at high risk of osteoporotic fracture the WHO developed the Fracture Risk Assessment (FRAX) tool (www.shef.ac.uk./FRAX). It is an internet based clinical tool for calculation of fracture risk in the individual patient based on assessment of significant risk factors for osteoporotic fracture. The FRAX algorithm is based on large-scale prospective population-based studies which isolated the following risk factors as significant determinants of fracture risk: age, gender, body weight and body mass index, a history of fracture, hip fracture in parents, current smoking, excessive alcohol intake, rheumatoid arthritis, glucocorticoid use, and other forms of secondary osteoporosis (Table 2) [13].
The National Osteoporosis Foundation (NOF) in the US and the National Osteoporosis Society (NOS) in the UK have integrated FRAX and BMD for case finding of individuals at high risk for fracture and for treatment decisions in their new guidelines. Treatment thresholds were put at 10-year fracture risk estimates from the FRAX algorithm, at which fracture prevention became cost-effective. Generally FRAX based ten year risks of 20% or higher for all osteoporotic fractures and 3% or higher for hip fracture are considered reasonable intervention thresholds [12, 13].
FRAX identifies patients at increased risk of osteoporotic fracture based on some of the dominant risk factors, but cannot be used in isolation. Several known determinant of fracture risk are, however, not included in FRAX. The algorithm does not take into account well known “dose effects” like glucocorticoid dose. Incorporation of BMD results is limited to results of BMD in the femoral neck. However, total hip BMD is a more precise measure and can be used interchangeably with femoral neck BMD in women, but not in men. Vitamin D deficiency, a well established risk factor for falls and hip fracture is not included. The same holds for bone markers, which have been shown to independently affect fracture risk. FRAX may also underestimate fracture risk in individuals with increased propensity for falls. More than 80% of women and men presenting with a clinical fracture to the emergency unit have one or more fall-related risks and exhibit a fourfold increased risk of falls in the year leading up to admission. In another study on 5- and 10-year absolute risks for fractures in patients using glucocorticoids, a history of falls had a greater impact on fracture risk than any other evaluated risk [26]. Finally it is important to remember that FRAX is only applicable in untreated patients. It cannot be used as a helper in decision making in patients, who already receive specific osteoporosis treatment. A recent study from Switzerland used FRAX to identify patient profiles with increased probability of fracture beyond currently accepted reimbursement thresholds for BMD and osteoporosis. The study found that in particular age, BMI and parental history of fracture increased the risk fo fracture substantially [27].
In patients with BMD-based osteoporosis or presenting with a clinical fracture or both, diagnostic evaluation is necessary to exclude secondary osteoporosis. Such evaluations should include hematologic parameters (Hb, WBC), serum 25-(OH)D3, calcium, creatinine, thyroid-stimulating hormone, parathyroid hormone (PTH), serum/urine electrophoresis and testosterone (in me). According to the clinical picture and suspicion, other serum measurements such as plasma cortisol, tests for celiac disease and selected other evaluations looking for secondary causes are indicated [28]. It is generally considered that secondary causes of osteoporosis are more common in men than women. Among secondary causes, hypogonadism resulting from the treatment of breast cancer with aromatase inhibitors use of androgen deprivation therapies in have become an emerging clinical problem [29].
There is general consensus on the need for specific osteoporosis treatment in patients with spine or hip fractures and low BMD. For other nonvertebral fractures different societies advocate different strategies. The NOS recommends drug treatment in all postmenopausal women with a history of any fragility fracture [12], while the NOF advocates performing a dual-energy x-ray absorptiometry (DXA) on patients after nonvertebral fractures to decide, whether specific osteoporotic therapy is indicated. Drug treatment should then be considered in patients having osteoporosis and in patients with osteopenia when FRAX indicates a 10-year fracture probability of at least 3% for hip or at least 20% for major fractures [9].
1.2 Treatment options
1.2.1 Lifestyle changes
General changes in life style like smoking cessation, regular exercise and optimization of nutirition should be implemented in all osteopenic patients. Patient compliance with these measures is, however, poor, and very few prospective data on the anti-fracture efficacy of such measures exist.. Smoking has emerged as a significant risk factor for fracture in many epidemiological studies [30–32], albeit the influence of dose and duration is less well defined. The same holds for exercise [33, 34], but exercise can slow down bone loss after menopause and is important for muscular strength and coordination in the elderly [32]. The impact of poor nutrition on skeletal health is apparent in its most extreme form in anorexia nervosa, where significant improvement of skeletal mass is important without a reversal of caloric intake in these young women [35, 36].
In recent years, vitamin D deficiency has emerged as a very important risk factor for osteoporotic fracture, especially at the hip. High turnover bone loss due to secondary hyperparathyroidism due to vitamin D deficiency is considered a major pathogenetic factor in senile osteoporosis [37]. Vitamin D deficiency is endemic worldwide [38] and patients with hip fracture generally have the lowest vitamin D levels among all patient groups studied [39, 40]. Vitamin D deficiency does not only cause weaker bones due to osteomalacia, but also severe myopathy with loss of muscle strength, selective loss of the rapid type-2 fibres, dyscoordination and consequently increased propensity for falls [41]. It is therefore not surprising that meta-analyses indicate that correction of vitamin D deficiency results in a decreased fall and fracture risk [42, 43], but the effects depend on the dose of vitamin D and the target population [44]. It is still a matter of debate which doses of vitamin D3 or D2 supplementation are necessary/optimal, taking into account baseline vitamin D status and the desired serum levels to be achieved by supplementation [31–33]. Daily intake of 400 IU/day is not sufficient, while 800 IU/day reduce falls and fractures significantly [42, 43]. In a recent controlled clinical trial Bischoff-Ferrari et al. demonstrated that in a population of post hip fracture patients maybe even higher doses are warranted. In this study a dose of 2,000 IU/day of D3 was superior to 800 IU/day in a cohort of 176 patients all undergoing moderate physiotherapy. Over a 1 year period the dose of 200 IU resulted in 25% less falls, 39% less readmissions to hospital and a staggering 90% reduction in all cause infections, when compared to 800 IU per day [45].
Several reviews have emphasized the need of addition of calcium to vitamin D for fracture prevention and a dose of 1,000 to 1,200 mg/day was advocated [46]. Whether the calcium dose can get too high is still a matter of debate, but studies from one center published in 2008 reported that supplements of 1,000 mg calcium/day on top of a baseline intake of 800 mg/day increased the risk of vascular events including myocardial infarction in healthy postmenopausal women men [47, 48]. In this context, it is reassuring that, when intake of vitamin D3 is sufficient, the need for calcium intake is considered to be lower [49].
1.2.2 Prevention of falls and protection against fall trauma
Over 90% of hip fractures and all Colles fractures are caused by falls, mostly in house. Vitamin D supplements improve muscle function and decrease the risk of falls, as discussed above. The role of physical exercise is still debated, but exercise interventions together with other measures such as removing loose carpets, reduce use of sleep medicine and other tranquilizers, correct visual impairment etc. reduce the risk and rate of falls in older people living in the community [50], but no data that fall prevention decreases the risk of fracture are yet available. The role of hip protectors remains controversial. They seem to work in nursing homes [51, 52], but less in community dwelling elderly, mainly due to discomfort and practicality [53, 54].
1.2.3 Pharmacotherapy
The last 2 decades have seen the emergence of an ever growing list of effective and safe pharmacological agents for the treatment of osteoporosis. These therapies for the prevention and treatment of osteoporosis affect bone remodeling by either inhibiting bone resorption (antiresorptive or anti-activation drugs) or enhancing bone formation (anabolic regimens). Antiresorptive drugs do not lead to net accrual of bone in the skeleton, despite the fact that BMD is increased. They mainly act by reducing bone turnover (therefore the term anti-activation), thus reducing the impact of the negative remodeling balance present in osteoporotics [55] and limiting further deterioration of cancellous- and cortical bone structure [56]. These drugs also reduce the remodeling space and increase the degree by which bone matrix is mineralized, which is behind the increase in BMD and add to the antifracture efficacy of these agents. On average, these drugs increase DXA BMD by 2–10%, and this increase is mainly caused by reduced porosity of cortical and cancellous bone combined with the increased incorporation of bone mineral into bone matrix during low turnover. Antiresorptives belonging to the bisphosphonate group are the most widely used agents. These agents have shown significant efficacy in randomized trials reducing event rates by up to 70% for spine fractures, 40–50% for hip fractures and 20–25% for nonvertebral fractures overall. Clinical painful spine fractures show reductions of up to 85–90% [21, 57–59]. In hip fracture patients, treatment with iv.bisphosphonatea furthermore has demonstrated a significant 28% reduction of all cause mortality.
Anabolic drugs, of which only recombinant PTH are currently available, on the other hand add net bone mass to the skeleton and actually improve bone structure and increase bone size from the osteoporotic state [60, 61].
Despite the advances in pharmacotherapy the majority of patients with osteopenia and osteoporosis are not treated [50], and for patients who initiate therapy, adherence to therapy is commonly below 50% at 1 to 2 years [51].
1.3 Antiresorptive (anti-activation) drugs
1.3.1 Estrogen
Estrogen receptors have been demonstrated on both osteoblasts and osteoclasts [62, 63]. Estrogen replacement therapy (ERT) or combined estrogen/progestin therapy (HRT) reduces bone turnover by about 50% and improves bone balance at each invidual BMU in postmenopausal women [64]. The Women’s Health Initiative (WHI), a randomized study comprising over 16,000 postmenopausal women, demonstrated a significant 34% reduction of hip fractures after treatment with combined conjugated equine estrogen and [65] as well as estrogen alone in those women who had undergone hysterectomy [66]. The study, however, also found a nearly 30% increased risk of coronary heart disease, 40% increased risk of stroke, increased risk of thromboembolic events and 26–35% increased risk of breast cancer. These results led to less enthusiasm for long-term estrogen therapy world wide. The decision to initiate ERT/HRT should be individualized and based on a balanced assessment of risk and benefits by the physician and patient. Current recommendations support restricting the use of estrogen in most women to 5 years in the perimenopausal period [67], with the aim mainly to reduce hot flushes and other postmenopausal symptoms, and regular mammography should be performed.
1.3.2 Selective estrogen receptor modulators
Selective estrogen receptor modulators (SERMs) are non-steroidal synthetic agents, which exert estrogen-like properties on the bone and cardiovascular systems, but estrogen antagonistic actions in the breast and, in some cases, the endometrium. The first SERM developed both for breast cancer prevention and for osteoporosis, raloxifene, is now approved in many countries for the treatment of osteoporosis. This drug was tested in the pivotal Multiple Outcomes of Raloxifene Evaluation (MORE), a multicenter study of over 7,700 postmenopausal women with at least one vertebral fracture or osteoporosis on the basis of a T score of −2.5 or below. In this study a dose of 60 mg/day reduced vertebral fracture risk by 30% in patients with prevalent fracture and 52% in patients without prevalent fracture [68]. Similar to results obtained with another SERM mainly used in oncology, tamoxifen, the risk of invasive breast cancer was decreased by 72% during the MORE study [69, 70]. In some women hot flashes and other menopausal symptoms may recur on raloxifene. Similar to estrogen, raloxifene, increases the risk of deep venous thrombosis three-fold [68]. The STAR trial comparing raloxifene to Tamoxifen found reported that Raloxifene was as effective as tamoxifen in reducing the risk of invasive breast cancer and carried a lower risk of thromboembolic events and cataracts, but a non-statistically significant higher risk of non-invasive breast cancer. The risk of other cancers, fractures, ischemic heart disease, and stroke was similar for both drugs [71].
Other SERMs, like Bazodoxifene and Lasofoxifene, are currently under development.
Bazodoxifene decreases vertebral fracture risk to a degree similar to that of raloxifene (approximately 40% over a 3-year period [89]) and, in a post hoc analysis, reduced the risk of non-spine fractures in a subgroup of patients with high risk for fractures based on FRAX [1]. Lasofoxifene was studied in the Postmenopausal Evaluation And Risk reduction with Lasofoxifene (PEARL) trial. The drug caused significant reductions compared with placebo in vertebral and nonvertebral (but not hip) fracture risk as well as in estrogen receptor positive breast cancer with the 0.5 mg dose [72]. This is the only SERM, to date showing significant reduction of nonvertebral fractures as well as significant reductions in risk of stroke, cholesterol levels and cardiovascular disease [72, 73]. In this study, however, a small rise in overall mortality was reported in the 0.25 mg dose but not with the higher 0.5 mg dose.
1.3.3 Androgen replacement therapy in males
In hypogonadal males low testosterone levels result in a high turnover state in bone leading to bone loss and increased risk of fracture. The main driver of this turnover increase is low circulating estrogen levels, just as in postmenopausal women [74]. The low estrogen arises from insufficient aromatase conversion from testosterone, either due to low testosterone levels or insufficient aromatase activity [75]. Testosterone replacement therapy in hypogonadism will increase circulating estradiol levels and thereby reduce bone turnover and increase BMD [76]. In hypogonadism, usually defined as total testosterone levels below 8 nmol/l and hypogonadal symptoms [77], testosterone replacement will lead to increases in bone mass similar to those seen after ERT/HRT [77, 78], but randomized controlled studies with fracture end points are still lacking. Due to the fear of inducing prostate cancer clinicians have, however, been quite reluctant to institute testosterone replacement therapy. Recent data suggest, however, that prostate cancers occurring in hypogonadal males have a worse prognosis than cancers occurring in eugonadism,[79]. Moreover, 16 population studies were unable to demonstrate any relation between testosterone levels and risk kfo prostate cancer [80]. Nevertheless, regular controls of prostate specific antigen (PSA) and digitial rectal exploration before and after institution of therapy is still warranted.
1.3.4 Calcitonin
Both injectable and intranasal administration of calcitonin results either stabilization of BMD or small increases in spine BMD (1–3%) and reductions in bone turnover as reflected in decreased levels of bone markers [81]. A 5-year multicenter study of 1,255 postmenopausal women showed a 36% reduction in vertebral fractures in the 200 IU, but not in the two other dosage groups (100 and 400 IU). The study also had a dropout rate of around 50% [82]. Calcitonin has been shown to improve bone structure in the forearm [83]. Nasal calcitonin is generally well tolerated, with occasional rhinitis. Headache, flushing, nausea, and diarrhoea have been reported more commonly with subcutaneous, than with intranasal calcitonin. Due to the absence of significant reductions in hip- and nonvertebral fractures calcitonin is considered a second- or third-line agent for osteoporosis treatment in most countries.
1.3.5 Bisphosphonates
Bisphosphonates are potent inhibitors of bone resorption and reduce the risk of osteoporotic fractures when administered orally or by intravenous infusion [84]. They are simple molecules with a P-C-P backbone and variations in the structure of the amino side chains of these drugs determine their pharmacological activity. The most common bisphosphonates licensed and used internationally are alendronate, risedronate, ibandronate, and zoledronic acid. These drugs are used in osteoporosis, Paget disease. In higher doses (10–12 fold the doses used in osteoporosis) these drugs are used for the treatment of advanced metastatic cancer with bone involvement (e.g. multiple myeloma, breast and prostate cancer) and hypercalcemia of malignancy..
All oral bisphosphonates are poorly absorbed, with bioavailability of less than 1%. This limitation is overcome by iv administration. After absorption the drugs are primarily going to the skeleton or excreted via the kidneys. This results in absence of detectable bisphosphonate in the circulation 2–3 days after administration, while the drugs exhibit a variable but generally long skeletal retention (years to tens of years). Once adsorbed onto bone surfaces, the mechanism of action is based on two actions: 1) tight binding to hydroxyapatite crystals in bone; 2) inhibition of important metabolic pathways in osteoclasts after incorporation during resorption of bisphosphonate coated bone. The latter mechanism is either via accumulation of toxic ATP analogues for non-nitrogen containing bisphosphonates or inhibition of a key enzyme in the mevalonate pathway by nitrogen containing bisphosphonates impairing cholesterol metabolism of the osteoclast and leading to cytoskeletal alterations and premature osteoclast cell death via apoptosis [84].
Histomorphometric analyses of bone biopsies obtained from patients treated with bisphosphonates have revealed dose dependent reduction in bone turnover, without any adverse effects on osteoblastic function or matrix mineralization [85, 86]. A few studies have also demonstrated improved balance at the BMU-level [87].
Over prolonged administration, continued local inhibition of continuously deposited and recycled bisphosphonates may partially account for a lack of rapid loss of BMD gains, when these agents are discontinued [88], which is a unique property, compared to other osteoporosis treatments.
The main side effect of oral bisphosphonates is gastrointestinal (GI) intolerance, [89]. Most reported GI symptoms have been non-ulcer dyspepsia, but in most clinical trials, no significant differences between those treated with bisphosphonates and those receiving placebo have been demonstrable [89]. There have been rare reports of severe esophagitis in patients taking oral bisphosphonates [90].
Recently two purported adverse effects to long term bisphosphonate treatment have been reported and been subject to significant scrutiny: 1) osteonecrosis of the jaw (ONJ) 2) atypical femoral fractures. In relation to ONJ it is important to distinguish between patients receiving bisphosphonate therapy for advanced cancer with bone metastases and patients receiving the drug for the treatment of osteoporosis. In oncology only iv. Bisphosphonates are used with doses exceeding the doses used in osteoporosis by a factor of 10–12, and ONJ has been reported in an estimated 1–2% of cancer patients receiving higher doses of predominately intravenous bisphosphonates for patients with malignancies in particular [91]. Among malignancies the vast majority of ONJ cases are seen in myeloma and breast cancer. The underlying cause of this selectivity is still unknown. Cases also have been described in patients receiving bisphosphonates for osteoporosis, but are much rarer. Several large scale studies have estimated the risk in osteoporosis to e between 1/10,000 and 1/100,000 [92, 93]. Atypical femoral fractures have also been reported in several small case series and are mostly transverse fractures of the femoral shaft in patients on long term treatment with alendronate. The fracture event is usually preceded by longstanding pain in the affected hip and most fractures happen without fall-related trauma. Bilateral fractures may occur, and on x-rays, signs of periosteal reaction and diffuse or focal cortical thickening are usually seen [94]. Whether direct causality between bisphosphonate use and these fractures can ever be established is doubtful. In treatment naive patients atypical fractures constitute 4% of all hip fractures, and a large scale epidemiologic study from Denmark could not demonstrate any correlation to long term bisphosphonate use [95]. Currently, the general consensus is that the benefit of significant reduction of all hip fractures after bisphosphonate treatment far outweighs the small risk of an atypical fracture, but strategies are being developed in order to identify individuals at risk.
Alendronate was the first amino-bisphosphonate approved by the US Food and Drug Administration for the treatment and prevention of osteoporosis. The registration was based on daily dosing of 10 mg/day. The Fracture Intervention Trial (FIT) enrolled 2,027 older women with at least one prior vertebral fracture and low femoral neck BMD. In this group alendronate induced significant 47% and 51% reductions in morphometric vertebral and hip fractures, respectively [58]. In FIT subjects without prevalent vertebral fractures, alendronate 10 mg decreased radiographic vertebral fractures by 44% [8]. A multinational study of alendronate similarly identified a 47% risk reduction for nonvertebral fractures in women with low bone mass [96]. A long-term extension to the FIT study (FLEX) demonstrated the long term safety over a period of 10 years. With the exception of clinical vertebral fractures, fracture risk reduction at other skeletal sites was statistically indistinguishable in those receiving 5 years on followed by 5 years off of alendronate versus a full 10 years of therapy [88]. Later, however, once-weekly preparation of alendronate (70 mg) has greatly exceeded daily administration based on improved ease of use, and tolerability that is equivalent to or better than daily therapy [97].
The decision about whether to stop therapy with bisphosphonate after a finite period of time is subject to debate. Further analysis of the FLEX data revealed that women with a femoral neck BMD T score of −2.5 or below at the 5-year mark had a higher risk of subsequent fractures after discontinuation [98], so some centers have adopted strategies where bisphosphonate treatment is discontinued, only in patients where t-score is > −2,5.
Risedronate is a pyridinyl amino-bisphosphonate that increases bone mass and prevents fractures [99]. In two studies one US [59] and one multinational [100] VERT (Vertebral Efficacy with Risedronate Therapy) studies, 1,226 and 2,458 postmenopausal women with at least one prior vertebral fracture were were enrolled and randomized to treatment with 5 mg of risedronate or Ca + D alone. Women receiving risedronate exhibited 41–49% reduction in vertebral fractures and 33–39% reduction in nonvertebral fractures. The drug was also tested In the Hip Intervention Program study, where risedronate 5 mg significantly reduced hip fractures among women with confirmed low bone mass but not among those selected primarily on the basis of fall risks without documented osteoporosis [101].
Ibrandronate either orally (daily or monthly) or intravenously reduced markers of bone turnover, increased BMD [102], and reduced fractures of the vertebrae by 52% [103]. In the pivotal fracture study no significant reduction of hip fractures was seen, but post hoc analyses claimed significant reductions in individuals with baseline BMD t-scores at or below −3.0.
Zoledronic acid is administered as a yearly 5 mg intravenous infusion and significantly reduced both vertebral (by 70%) hip (by 41%) and non-vertebral fractures (by 25%) in postmenopausal women with osteoporosis [5]. The drug was also tested in women and men after surgical repair of a hip fracture, and in this population a significant reduction in subsequent clinical fractures along with a reduction in mortality was seen [10]. As with other iv. Bisphosphonates the main side effects include a post-dose response, characterized by fever, myalgia and arthralgia in about 15–20% of patients. These symptoms are seen mainly after administration of the first dose, but rarely with subsequent dosing, and seem to be less pronounced in patients previously treated with oral bisphosphonates. Due to the higher Cmax achieved with iv bisphosphonates the risk of renal impairment is increased over oral adminsitration. However, if a minimum infusion time of 15 min is adhered to. and a baseline creatinine clearance of 35 ml/min is used at cut off for institution of treatment, no increase in renal side effects over placebo are seen [104].
1.4 Effects of bisphosphonates in osteopenia
The FIT II study studied 8,704 women with low fermoral neck BMD, but no prevalent vertebral fractures, who were randomized to alendronate (5–10 mg) or placebo for a period of 4 years. The primary endpoint was reduction of all clinical fractures (spine, hip, nonvertebral) [8]. 63% of women exhibited t-scores in the osteopenic range (in this study t-scores between −1,6 and −2,5): Alendronate did not significantly reduce clinical fractures in women with baseline t-scores in the osteopenic range, but induced significant reduction (44%) of morphometric vertebral fractures. The NNT to prevent 1 vertebral fracture ranged between 59 and 363 in the lower and higher osteopenic range, respectively [8]. The FOSIT study evaluated the safety and effects on bone mineral density (BMD) of alendronate 10 mg in postmenopausal women with lumbar spine BMD t-score of −2 or more. After 12 months the incidence of nonvertebral fractures was reduced significantly by 47% [96].
1.4.1 Parathyroid hormone
Continuous excessive secretion of parathyroid hormone (PTH) (e.g. primary hyperparathyroidism) causes bone catabolism, characterized by cortical bone loss in particular [105]. In contrast, exogenously administered intermittent PTH leading to a peak of excess PTH for about 3 h is anabolic due to stimulation of osteoblastic bone formation and increased skeletal remodeling activity. Daily administration of the approved dose of 20 ug of PTH fragment [1–34] (teriparatide) increased BMD by an average of 9.7% in human studies [61, 106, 107] and significantly decreased the risk of vertebral (65% risk reduction in those on 20 ug/day) and nonvertebral fractures (35% with 20 ug/day) and increased BMD at all sites investigated, except for the radial shaft [61]. Nonvertebral fragility fractures were reduced by 53%. The initial decrease at this site, coincides with the maximal increase in cortical porosity seen with the drug at 6 months [108]. Teriparatide increases cancellous bone volume, restores trabecular bone architecture and increases cortical thickness. It also leads to periosteal new bone formation and increases cross-sectional area, potentially increasing cortical bone strength [109]. These are specific cortical effects, which are not seen with anti-resorptive drugs, and they may be responsible for the pronounced reduction of non-vertebral fractures by up to 80% in patients treated for more than 18 months in a post hoc analysis of clinical fractures in the pivotal PTH trial [110].
Full length PTH [1–84] has also been tested as a treatment modality for osteoporosis. The dose used was 100 ug/Day, which is equivalent to a PTH [1–34] dose of 40 ug. In a study on 2,532 postmenopausal women, PTH[1–84] increased spine and hip BMD by 6,9 and 2,1%, respectively, and reduced the risk for new or worsened vertebral fractures by 40%. No reduction of nonvertebral fractures was demonstrable [111].
PTH [1–34] and PTH [1–84] have both been associated with nausea, and headache. PTH [1–34] 20 ug/day may lead to asymptomatic mild hypercalcemia, a side effect seen more frequently with PTH [1–84], where dose reduction regimens had to be instituted in a significant number of patients in order to avoid symptomatic hypercalcemia [111]. Clinical trials of teriparatide were terminated early by the finding of osteosarcoma in Fisher rats [61], and similar effects were seen with PTH [1–84]. However after widespread use in humans no significant increase in osteosarcoma cases beyond the background rate has been detected. Due to this finding in the initial trial and in repeated toxicology trials in rats, PTH is recommended for treatment for a limited period of 24-months.
There is an enhanced effect on bone mass when PTH is sequentially followed by antiresorptives like alendronate [112] or estrogen [113], most probably due to a reduction of the remodeling space brought about by these agents. If some kind of antiresorptive treatment is not given, bone mass will return to baseline within 2 years after discontinuation of PTH, albeit anti-fracture efficacy seems preserved [114]. Oral bisphosphonates started prior to or concurrently with PTH may attenuate bone mass improvement and bone marker increases seen with PTH alone. Iv. Bisphosphonates seem to be causing less blunting of the BMD and marker response [115].
PTH is currently used most commonly in adults with severe osteoporosis,—many of whom have had fractures while on other antiosteoporotic agents or have had intolerance to bisphosphonates, and will rarely have indications in osteopenic patients.
1.4.2 Denosumab
The discovery of the receptor activator of the nuclear factor kappa B ligand RANKL/RANK/osteoprotegerin (OPG) pathway regulating osteoclastic differentiation has defined new targets for inhibition of osteoclastic bone resorption. Denusomab, a specific recombinant humanized monoclonal antibody against RANKL, is the most effective suppressor of bone resorption yet developed. It reduces bone turnover by over 95% [116]. Consequently the BMD increases seen are more pronounced than with other antiresorptives. In the pivotal FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months) study of nearly 8,000 women [21], denosumab reduced the risk of new radiographic vertebral fracture by 68%, with a cumulative incidence of 2.3% in the Denosumab group versus 7.2% in the placebo group. Denosumab significantly reduced the risk of hip and nonvertebral fracture by 40% and 20%, respectively. In clinical trials with Denosumab, overall adverse events were similar to placebo. There was no increase in the risk of cancer, infection, cardiovascular disease, delayed fracture healing, or hypocalcemia. In the osteoporosis trials with Denosumab there were no cases of osteonecrosis of the jaw. In the oncology trials with Denosumab, however, the risk of ONJ was found to be similar to that of Zoledronic acid [117].
1.4.3 Strontium ranelate
Daily intake of strontium ranelate has been shown to reduce the risk of vertebral and nonvertebral fractures in postmenopausal women with osteoporosis or a prevalent vertebral fracture or both. The exact MOA of strontium ranelate is still poorly defined, however, but the drug increases matrix mineralization and reduce spine fractures by 38% after 3 years [118, 119]. In the pivotal trial on nonvertebral fractures no significant overall reduction of hip fracture was seen, but in a high risk subgroup (women over 74 years old with low BMD at the femoral neck), a significant reduction of 36% was seen [119]. Other post hoc analyses have demonstrated efficacy in women with osteopenia and women older than 80 years [120].
2 Summary
An ever increasing array of effective treatments is at our disposal, to protect patients with osteopenia against fractures. While there is general consensus on treating osteopenic individuals with prevalent low energy fractures, the treatment of osteopenia without fracture is still debatable. However, current evidence indicates that specific pharmacotherapy should be instituted if an osteopenic patients has prevalent fractures or suffers new fractures, be it clinical or asymptomatic. Moreover, a significant accumulation of several significant risk factors, for example as indicated by the FRAX tool may constitute an indication for pharmacotherapy. Patients without such risk factors should be counselled on a “bone friendly” lifestyle with nutritional modifications, regular exercise, moderation in alcohol use and If possible smoking cessation. In patients with low vitamin D levels, Ca + D supplementation may also be indicated (Fig. 3).
Amino-bisphosphonates, taken orally or intravenously, remain the dominant treatment modalities for osteoporosis. They reduce fracture risk in osteoporotic as well as osteopenic individuals. Questions exist about the very-long-term safety of these drugs, but the best data available so far [88], suggest that 10 years with 90% suppression of bone turnover is safe. Denosumab constitutes a future alternative to bisphosphonates. In younger postmenopausal women with osteopenia, estrogen or estrogen/progestin still has a place as a short term (up to 5 years) treatment, especially in women with menopausal symptoms. Similarly SERMs should be considered in younger postmenopausal women, especially those at increased risk of breast cancer. In males with low testosterone levels, testosterone substitution is indicated as it improves skeletal integrity. We still need long term controlled studies on this treatment, but the risk of prostate cancer does not seem to be as big as previously anticipated. Teriparatide, would currently rarely be considered in women or men with cheaper anabolics available, however, initial therapy with anabolics to bring osteopenic patients out of the risk zone followed by an antiresorptive would probably be the ideal treatment.
References
Siris ES, Chen YT, Abbott TA, Barrett-Connor E, Miller PD, Wehren LE, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108–12.
Ensrud KE, Thompson DE, Cauley JA, Nevitt MC, Kado DM, Hochberg MC, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture intervention trial research group.[see comment]. J Am Geriatr Soc. 2000;48:241–9.
Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15:38–42.
Quandt SA, Thompson DE, Schneider DL, Nevitt MC, Black DM, Fracture Intervention Trial Research Group. Effect of alendronate on vertebral fracture risk in women with bone mineral density T scores of−1.6 to −2.5 at the femoral neck: the fracture intervention trial. Mayo Clinic Proc. 2005;80:343–9.
Black DM, Boonen S, Cauley J, Delmas P, Eastell R, Reid I, et al. Effect of once-yearly infusion of zoledronic acid 5 mg on spine and hip fracture reduction in postmenopausal women with osteoporosis: the HORIZON pivotal fracture trial. Arthritis Rheum. 2006;54:S305.
Riancho JA. Number of patients to be treated and number of prevented fractures: clinical efficiency of osteoporosis treatment with diphosphonate alendronate. Rev Clin Esp. 1999;199:349–55.
Lamy O, Krieg MA. Effects of different treatments for osteoporosis on prevention of fractures: toward and individualized and economic approach. Praxis (Bern 1994). 2004;93:399–405.
Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial.[see comment]. JAMA. 1998;280:2077–82.
National osteoporosis Foundation-Clinicans guide to prevention and treatment of osteoporosis. www.nof.org/professionals/Clinicians_Guide.htm [serial online] Available at: www.nof.org/professionals/Clinicians_Guide.htm.
Lyles KW, Colon-Emeric C, Magaziner J, Adachi J, Pieper CF, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Lavecchia C, Zhang J, Mesenbrink P, Abrams K, Orloff JJ, Horowitz Z, Eriksen EF, Boonen S. The effect of once yearly zoledronic acid on new fractures and mortality after hip fracture. 2007; In press.
van Helden S, van Geel AC, Geusens PP, Kessels A, Nieuwenhuijzen Kruseman AC, Brink PR. Bone and fall-related fracture risks in women and men with a recent clinical fracture. J Bone Joint Surg Am. 2008;90:241–8.
Kanis JA, McCloskey EV, Johansson H, Strom O, Borgstrom F, Oden A. Case finding for the management of osteoporosis with FRAX—assessment and intervention thresholds for the UK. Osteoporos Int. 2008;19:1395–408.
Dawson-Hughes B, Tosteson AN, Melton LJ 3rd, Baim S, Favus MJ, Khosla S, Lindsay RL. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19(4):449–58.
van Geel TA, van Helden S, Geusens PP, Winkens B, Dinant GJ. Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis. 2009;68:99–102.
Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–21.
Chevalley T, Hoffmeyer P, Bonjour JP, Rizzoli R. An osteoporosis clinical pathway for the medical management of patients with low-trauma fracture. Osteoporos Int. 2002;13:450–5.
Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–8.
Lems WF. Clinical relevance of vertebral fractures. Ann Rheum Dis. 2007;66:2–4.
Delmas PD, Genant HK, Crans GG, Stock JL, Wong M, Siris E, et al. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone. 2003;33:522–32.
Black DM, Boonen S, Cauley J, Delmas P, Eastell R, Reid I, et al. Effect of once-yearly infusion of zoledronic acid 5 mg on spine and hip fracture reduction in postmenopausal women with osteoporosis: the HORIZON pivotal fracture trial. J Bone Miner Res. 2006;21:S16.
Cummings SR, San MJ, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65.
Schousboe JT, Vokes T, Broy SB, Ferrar L, McKiernan F, Roux C, et al. Vertebral fracture assessment: the 2007 ISCD official positions. J Clin Densitom. 2008;11:92–108.
Delmas PD, van de Langerijt L, Watts NB, Eastell R, Genant H, Grauer A, et al. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT study. J Bone Miner Res. 2005;20:557–63.
Gallacher SJ, Gallagher AP, McQuillian C, Mitchell PJ, Dixon T. The prevalence of vertebral fracture amongst patients presenting with non-vertebral fractures. Osteoporos Int. 2007;18:185–92.
Netelenbos JC, Lems WF, Geusens PP, Verhaar HJ, Boermans AJ, Boomsma MM, et al. Spine radiographs to improve the identification of women at high risk for fractures. Osteoporos Int. 2009;20:1347–52.
van Staa TP, Geusens P, Pols HA, de Laet C, Leufkens HG, Cooper C. A simple score for estimating the long-term risk of fracture in patients using oral glucocorticoids. QJM. 2005;98:191–8.
Lippuner K, Johansson H, Kanis JA, Rizzoli R. FRAX assessment of osteoporotic fracture probability in Switzerland. Osteoporos Int. 2010;21:381–9.
Tannenbaum C, Clark J, Schwartzman K, Wallenstein S, Lapinski R, Meier D, et al. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab. 2002;87:4431–7.
Saad F, Adachi JD, Brown JP, Canning LA, Gelmon KA, Josse RG, et al. Cancer treatment-induced bone loss in breast and prostate cancer. J Clin Oncol. 2008;26:5465–76.
Orwoll ES, Bevan L, Phipps KR. Determinants of bone mineral density in older men. Osteoporos Int. 2000;11:815–21.
Bjarnason NH, Christiansen C. The influence of thinness and smoking on bone loss and response to hormone replacement therapy in early postmenopausal women. J Clin Endocrinol Metab. 2000;85:590–6.
Cummings SR. Prevention of hip fractures in older women: a population-based perspective. Osteoporos Int. 1998;8 Suppl 1:S8–12.
Chesnut III CH. Bone mass and exercise. [Review] [17 refs]. Am J Med. 1993;95:34S–6.
Turner CH, Robling AG. Exercise as an anabolic stimulus for bone. [Review] [89 refs]. Curr Pharm Des. 2004;10:2629–41.
Davies KM, Pearson PH, Huseman CA, Greger NG, Kimmel DK, Recker RR. Reduced bone mineral in patients with eating disorders. Bone. 1990;11:143–7.
Vestergaard P, Emborg C, Stoving RK, Hagen C, Mosekilde L, Brixen K. Patients with eating disorders. A high-risk group for fractures. Orthop Nurs. 2003;22:325–31.
Riggs BL. Role of the vitamin D-endocrine system in the pathophysiology of postmenopausal osteoporosis. [Review] [41 refs]. J Cell Biochem. 2003;88:209–15.
Kuchuk NO, van Schoor NM, Pluijm SM, Chines A, Lips P. Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: global perspective. J Bone Miner Res. 2009;24:693–701.
Bischoff-Ferrari HA, Can U, Staehelin HB, Platz A, Henschkowski J, Michel BA, et al. Severe vitamin D deficiency in Swiss hip fracture patients. Bone. 2008;42:597–602.
Chapuy MC, Schott AM, Garnero P, Hans D, Delmas PD, Meunier PJ. Healthy elderly French women living at home have secondary hyperparathyroidism and high bone turnover in winter. EPIDOS study group. J Clin Endocrinol Metabol. 1996;81:1129–33.
Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Andersen H, et al. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int. 2000;66:419–24.
Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, wson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–64.
Bischoff-Ferrari HA, wson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, et al. Effect of Vitamin D on falls: a meta-analysis. JAMA. 2004;291:1999–2006.
Izaks GJ. Fracture prevention with vitamin D supplementation: considering the inconsistent results. BMC Musculoskelet Disord. 2007;8:26.
Bischoff-Ferrari H, et al. Effects of extended physiotherapy and high dose vitamin D on falls and morbiidy after hip fracture. Arch Intern Med. 2010;170(9):813–20.
Boonen S, Lips P, Bouillon R, Bischoff-Ferrari HA, Vanderschueren D, Haentjens P. Need for additional calcium to reduce the risk of hip fracture with vitamin d supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J Clin Endocrinol Metab. 2007;92:1415–23.
Bolland MJ, Barber PA, Doughty RN, Mason B, Horne A, Ames R, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ. 2008;336:262–6.
Reid IR, Ames R, Mason B, Reid HE, Bacon CJ, Bolland MJ, et al. Randomized controlled trial of calcium supplementation in healthy, nonosteoporotic, older men. Arch Intern Med. 2008;168:2276–82.
Heaney RP. The Vitamin D requirement in health and disease. J Steroid Biochem Mol Biol. 2005;97:13–9.
Gillespie LD, Robertson MC, Gillespie WJ, Lamb SE, Gates S, Cumming RG, Rowe BH. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009:CD007146.
Forsen L, Arstad C, Sandvig S, Schuller A, Roed U, Sogaard AJ. Prevention of hip fracture by external hip protectors: an intervention in 17 nursing homes in two municipalities in Norway. Scand J Public Health. 2003;31:261–6.
Meyer G, Warnke A, Bender R, Muhlhauser I. Effect on hip fractures of increased use of hip protectors in nursing homes: cluster randomised controlled trial. BMJ. 2003;326:76.
Cameron ID, Venman J, Kurrle SE, Lockwood K, Birks C, Cumming RG, et al. Hip protectors in aged-care facilities: a randomized trial of use by individual higher-risk residents. Age Ageing. 2001;30:477–81.
O’Halloran PD, Murray LJ, Cran GW, Dunlop L, Kernohan G, Beringer TR. The effect of type of hip protector and resident characteristics on adherence to use of hip protectors in nursing and residential homes–an exploratory study. Int J Nurs Stud. 2005;42:387–97.
Eriksen EF, Hodgson SF, Eastell R, Cedel SL, O’Fallon WM, Riggs BL. Cancellous bone remodeling in type I (postmenopausal) osteoporosis: quantitative assessment of rates of formation, resorption, and bone loss at tissue and cellular levels.[see comment]. J Bone Miner Res. 1990;5:311–9.
Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. [Review] [63 refs]. J Bone Miner Res. 2005;20:177–84.
Delmas P, Munoz F, Black D, Cosman F, Boonen S, Watts N, Kendler D, Eriksen E, Mesenbrink P, Eastell R. Effects of yearly zoledronic acid 5 mg on bone turnover markers and relation of PINP with fracture reduction in postmenopausal women with osteoporosis. J Bone Miner Res. 2009.
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture intervention trial research group.[see comment]. Lancet. 1996;348:1535–41.
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group.[see comment]. JAMA. 1999;282:1344–52.
Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone [1–28] [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18:1932–41.
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis.[see comment]. N Engl J Med. 2001;344:1434–41.
Eriksen EF, Colvard DS, Berg NJ, Graham ML, Mann KG, Spelsberg TC, et al. Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 1988;241:84–6.
Oursler MJ, Osdoby P, Pyfferoen J, Riggs BL, Spelsberg TC. Avian osteoclasts as estrogen target cells. Proc Natl Acad Sci USA. 1991;88:6613–7.
Eriksen EF, Langdahl B, Vesterby A, Rungby J, Kassem M. Hormone replacement therapy prevents osteoclastic hyperactivity: a histomorphometric study in early postmenopausal women. J Bone Miner Res. 1999;14:1217–21.
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33.
The womens health initiative steering committee effects of conuated equine estrogen in posstmenopausal women with hysterectomy: the womens health initiative randomized controlled trial. JAMA [serial online].
Nelson HD, Helfand M, Woolf SH, Allan JD. Screening for postmenopausal osteoporosis: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:529–41.
Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators.[see comment][erratum appears in JAMA 1999 Dec 8;282(22):2124]. JAMA. 1999;282:637–45.
Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple outcomes of raloxifene evaluation. JAMA. 1999;281:2189–97.
Cauley JA, Norton L, Lippman ME, Eckert S, Krueger KA, Purdie DW, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res Treat. 2001;65:125–34.
Vogel VG, Constantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727–41.
Cummings SR, Ensrud K, Delmas PD, LaCroix AZ, Vukicevic S, Reid DM, et al. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med. 2010;362:686–96.
McClung MR, Siris E, Cummings S, Bolognese M, Ettinger M, Moffett A, et al. Prevention of bone loss in postmenopausal women treated with lasofoxifene compared with raloxifene. Menopause. 2006;13:377–86.
Khosla S, Melton III LJ, Riggs BL. Clinical review 144: estrogen and the male skeleton. [Review] [54 refs]. J Clin Endocrinol Metab. 2002;87:1443–50.
Carlsen CG, Soerensen TH, Eriksen EF. Prevalence of low serum estradiol levels in male osteoporosis. Osteoporos Int. 2000;11:697–701.
Anderson FH, Francis RM, Peaston RT, Wastell HJ. Androgen supplementation in eugonadal men with osteoporosis: effects of 6 months’ treatment on markers of bone formation and resorption. J Bone Miner Res. 1997;12:472–8.
Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995–2010.
Amory JK, Watts NB, Easley KA, Sutton PR, Anawalt BD, Matsumoto AM, et al. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab. 2004;89:503–10.
Morgentaler A. Rapidly shifting concepts regarding androgens and prostate cancer. Sci World J. 2009;9:685–90.
Morgentaler A. Testosterone replacement therapy and prostate risks: where’s the beef? Can J Urol. 2006;13 Suppl 1:40–3.
Overgaard K, Hansen MA, Jensen SB, Christiansen C. Effect of salcatonin given intranasally on bone mass and fracture rates in established osteoporosis: a dose-response study. BMJ.
Chesnut CH, 3rd, Silverman S, Andriano K, Genant H, Gimona A, Harris S, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med. 2000;109:267–76
Chesnut CH, 3rd, Majumdar S, Newitt DC, Shields A, Van Pelt J, Laschansky E, et al. Effects of salmon calcitonin on trabecular microarchitecture as determined by magnetic resonance imaging: results from the QUEST study. J Bone Miner Res. 2005;20(9):1548–61.
Rogers MJ, Frith JC, Luckman SP, Coxon FP, Benford HL, Monkkonen J, et al. Molecular mechanisms of action of bisphosphonates. [Review] [69 refs]. Bone. 1999;24:73S–9.
Meunier PJ, Arlot M, Chavassieux P, Yates AJ. The effects of alendronate on bone turnover and bone quality. Int J Clin Pract Suppl. 1999;101:14–7.
Eriksen EF, Melsen F, Sod E, Barton I, Chines A. Effects of long-term risedronate on bone quality and bone turnover in women with postmenopausal osteoporosis. Bone. 2002;31:620–5.
Steiniche T, Hasling C, Charles P, Eriksen EF, Melsen F, Mosekilde L. The effects of etidronate on trabecular bone remodeling in postmenopausal spinal osteoporosis: a randomized study comparing intermittent treatment and an ADFR regime. Bone. 1991;12:155–63.
Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, et al. Effects of continuing or stopping alendronate after 5 years of treatment - The Fracture Intervention Trial long-term extension (FLEX): a randomized trial. JAMA. 2006;296:2927–38.
Bauer DC, Black D, Ensrud K, Thompson D, Hochberg M, Nevitt M, et al. Upper gastrointestinal tract safety profile of alendronate: the fracture intervention trial. Arch Intern Med. 2000;160:517–25.
de Groen PC, Lubbe DF, Hirsch LJ, Daifotis A, Stephenson W, Freedholm D, et al. Esophagitis associated with the use of alendronate. N Engl J Med. 1996;335:1016–21.
Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, Nooka A, Sayegh G, Guarneri V, Desrouleaux K, Cui J, Adamus A, Gagel RF, Hortobagyi GN. The frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008.
Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–91.
Felsenberg D. Osteonecrosis of the jaw—a potential adverse effect of bisphosphonate treatment. Nat Clin Pract Endocrinol Metabol. 2006;2:662–3.
Lenart BA, Lorich DG, Lane JM. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med. 2008;358:1304–6.
Abrahamsen B, Eiken P. Subtrochanteric femur fracture in patients treated with oral bisphosphonates: a national register study. Calcif Tissue Int. 2008;82:S209.
Pols HA, Felsenberg D, Hanley DA, Stepan J, Munoz-Torres M, Wilkin TJ, et al. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int. 1999;9:461–8.
Schnitzer T, Bone HG, Crepaldi G, Adami S, McClung M, Kiel D, et al. Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Aging Clin Exp Res. 2000;12:1–12.
Schwartz AV, Bauer DC, Cauley JA, Ensrud KE, Palermo L, Cummings SR, Black DM. Efficacy of continued alendronate for fractures in women without prevalent vertebral fracture: the FLEX Trial (abstract)., 22 ed. 2007;p. S1057.
Mortensen L, Charles P, Bekker PJ, DiGennaro J, Johnston Jr CC. Risedronate increases bone mass in an early postmenopausal population: two years of treatment plus one year of follow-up. J Clin Endocrinol Metab. 1998;83:396–402.
Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83–91.
McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group.[see comment]. N Engl J Med. 2001;344:333–40.
Riis BJ, Ise J, von Stein T, Bagger Y, Christiansen C. Ibandronate: a comparison of oral daily dosing versus intermittent dosing in postmenopausal osteoporosis. J Bone Miner Res. 2001;16:1871–8.
Chesnut CH, Ettinger MP, Miller PD, Baylink DJ, Emkey R, Harris ST, et al. Ibandronate produces significant, similar antifracture efficacy in North American and European women: new clinical findings from BONE. Curr Med Res Opin. 2005;21:391–401.
Boonen S, Sellmeyer DE, Lippuner K, Orlov-Morozov A, Abrams K, Mesenbrink P, et al. Renal safety of annual zoledronic acid infusions in osteoporotic postmenopausal women. Kidney Int. 2008;74:641–8.
Eriksen EF. Primary hyperparathyroidism: lessons from bone histomorphometry. [Review] [19 refs]. J Bone Miner Res. 2002;17 Suppl 2:N95–7.
Dobnig H, Turner RT. The effects of programmed administration of human parathyroid hormone fragment (1–34) on bone histomorphometry and serum chemistry in rats. Endocrinology. 1997;138:4607–12.
Orwoll E, Scheele W, Paul S, Adami S, Syversen U, ez-Perez A, et al. The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18:9–17.
McClung MR, San MJ, Miller PD, Civitelli R, Bandeira F, Omizo M, et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005;165:1762–8.
Zanchetta JR, Bogado CE, Ferretti JL, Wang O, Wilson MG, Sato M, et al. Effects of teriparatide [recombinant human parathyroid hormone (1–34)] on cortical bone in postmenopausal women with osteoporosis. J Bone Miner Res. 2003;18:539–43.
Lindsay R, Miller P, Pohl G, Glass EV, Chen P, Krege JH. Relationship between duration of teriparatide therapy and clinical outcomes in postmenopausal women with osteoporosis. Osteoporos Int. 2009;20:943–8.
Greenspan SL, Bone HG, Ettinger MP, Hanley DA, Lindsay R, Zanchetta JR, et al. Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146:326–39.
Rittmaster RS, Bolognese M, Ettinger MP, Hanley DA, Hodsman AB, Kendler DL, et al. Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate.[see comment]. J Clin Endocrinol Metab. 2000;85:2129–34.
77Cosman F, Nieves J, Woelfert L, Formica C, Gordon S, Shen V, et al. Parathyroid hormone added to established hormone therapy: effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res. 2001;16:925–31.
Lindsay R, Scheele WH, Neer R, Pohl G, Adami S, Mautalen C, et al. Sustained vertebral fracture risk reduction after withdrawal of teriparatide in postmenopausal women with osteoporosis. Arch Int Med. 2004;164:2024–30.
Cosman F, Eriksen EF, Recknor C, Miller P, Greenspan S, Papanastasiou P, Rao HV, Gasser J, Bucci-Recthweg C, Boonen S. Effects of once-yearly zoledronic acid 5 mg in combination with teriparatide (PTH) on postmenopausal women with osteoporosis. J Bone Miner Res. 2009.
Brown JP, Prince RL, Deal C, Recker RR, Kiel DP, de Gregorio LH, Hadji P, Hofbauer LC, varo-Gracia JM, Wang H, Austin M, Wagman RB, Newmark R, Libanati C, San MJ, Bone HG. Comparison of the effect of denosumab and alendronate on bone mineral density and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2009:1–34.
Amgen press release. http://wwwext.amgen.com/media/media_pr_detailjsp?year=2009&releaseID=1305355 [serial online].
Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis.[see comment]. N Engl J Med. 2004;350:459–68.
Reginster JY, Seeman E, De Vernejoul MC, Adami S, Compston J, Phenekos C, et al. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab. 2005;90:2816–22.
Seeman E, Vellas B, Benhamou C, Aquino JP, Semler J, Kaufman JM, et al. Strontium ranelate reduces the risk of vertebral and nonvertebral fractures in women eighty years of age and older. J Bone Miner Res. 2006;21:1113–20.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Eriksen, E.F. Treatment of osteopenia. Rev Endocr Metab Disord 13, 209–223 (2012). https://doi.org/10.1007/s11154-011-9187-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-011-9187-z