Abstract
Active carbon supported nickel catalysts prepared with nickel(II) nitrate and nickel(II) chloride precursors after reduction at 673 or 1173 K were investigated in the aqueous phase hydrodechlorination of 1,1,2-trichloroethene (TCE). These nickel catalysts facilitate the purification of water via decomposing TCE, but their catalytic properties depend on the chemistry of the nickel precursor used for Ni/C synthesis and pretreatment conditions. The chemistry of precursor catalysts affects the particle size, which in turn is correlated with catalytic activity. We observed the highest activity for Ni/C with the largest nickel particles. The application of high temperature reduction of nickel precursors increases the activity and minimizes the differences between the catalytic properties of the samples prepared from different salts.
Similar content being viewed by others
Introduction
The exceptional physicochemical properties of volatile chloroorganic compounds (Cl-VOCs) resulted in their widespread usage in many aspects of human activity. They are commonly used as solvents, additives in cleaning media, substrates in chemical syntheses and even anesthetics [1]. Paradoxically, the same desirable properties responsible for their popularity (high volatility and stability), caused their widespread and undesirable presence in the natural environment (air, soil and water). The contact with these compounds can be very dangerous for every living organism due to their carcinogenic, mutagenic and teratogenic properties [1]. As a result, many research groups all over the world have initiated studies aiming at solving the problems caused by the presence of Cl-VOCs in the world around us [2–4]. Among existing approaches catalytic hydrodechlorination is one of the most promising methods for this task [1]. Initially, the majority of studies in this field have focused on hydrodechlorination using noble metals as active phase and reagents supplied in gaseous phase [5, 6]. However, research transitioned relatively quickly to low-cost alternatives, which resulted in the utilization of variety of transition metals (e.g., nickel, copper) for this purpose [7–10]. Additionally, according to the fact that the most of detrimental substances release into the water, it is significant that interest in aqueous phase hydrodechlorination has been developed.
There are several criteria that should be fulfilled for the material that can be used as a support in the catalyst. Active carbons satisfied most of them. Relatively low price, high surface area, chemical resistance and inertness, both under acidic and basic conditions, makes activated carbon sufficient support [11]. Furthermore, the porosity and the surface can be manipulated in the manufacturing and activation processes [12]. Thanks to these properties, there is still growing interest in active carbons as the supports for metal catalysts in the hydrodechlorination reactions [9, 13].
The applicability of carbon supported nickel catalysts in the hydrodechlorination of 1,1,2-trichloroethene (TCE) reaction performed in the aqueous phase was demonstrated by Śrębowata et al. [2]. Catalyst prepared from NiCl2·6H2O by an incipient wetness impregnation of ordered mesoporous carbon showed good activity and selectivity in degradation of model Cl-VOCs.
Earlier studies clearly demonstrated the relationship between the selection of metal precursor used for catalyst synthesis and their catalytic behavior in the hydrodechlorination reaction performed in the gas phase [14, 15]. For example, Kim et al. [14] have chosen three commonly used nickel salts (nickel nitrate Ni(NO3)2, nickel chloride NiCl2 and nickel sulfate NiSO4) as precursors for γ-Al2O3 supported catalysts. These catalysts were then tested using model reaction of the gas phase hydrodechlorination of 1,1,2-trichloroethane. Physicochemical analysis showed that the properties, e.g., metal phase dispersion and strength of interaction between nickel and the support, of the obtained catalysts were strongly dependent on the chemical nature of the salts used. This resulted in differences in their catalytic activity, selectivity and stability. The Ni catalyst prepared using nickel nitrate exhibited the highest initial activity. However, it experienced severe catalyst deactivation and showed the lowest catalytic performance in the long run. After a period of catalytic reaction, this Ni catalyst with the highest nickel dispersion showed the lowest 1,1,2-trichloroethane conversion due to the drastic deactivation caused by the interaction between HCl and metallic nickel. The Ni–Cl and Ni–S catalysts were relatively resistant to deactivation because large nickel particles with a nickel aluminate structure in the Ni–Cl catalyst and highly dispersed sulfur species in the Ni–S catalyst prevented the strong interaction between HCl and metallic nickel.
A similar evaluation of catalytic activity of Ni deposited on Sibunit was performed in our research group recently [15]. In this study, we investigated the influence of other three different nickel precursors (chloride, nitrate, acetate) in hydrodechlorination of 1,2-dichloroethane in the gas phase. The correlation between catalysts the activity and the nickel salt used for preparation of Ni/Sibunit was very strong. The highest activity in 1,2-dichloroethane hydrodechlorination and the highest selectivity to the desired product (ethylene) were observed for the catalyst with the highest metal dispersion (the smallest particle size) prepared using nickel nitrate.
The catalyst activity depends not only on the type of metal precursor [14, 15], but also on the pretreatment conditions [13]. The role of this effect is one of the most common experimental variables. For example, Bonarowska et al. [16] investigated the catalytic activity of silica- and alumina-supported platinum catalysts in the gas phase hydrodechlorination of tetrachloromethane. A wide range (from 1.6 to 13.3 nm) of metal particle sizes in these catalysts was the consequence of the application of different calcination and reduction conditions, i.e., 573 K in oxygen for 2 h followed by reduction at 623 K led to the formation of very small Pt particles (1.6 nm). On the other hand the reduction at 673 K in the flow of wet H2 resulted in the formation of bigger metal particles. As a result, the correlation between the metal particle size and the catalytic behavior of platinum catalysts in the gas phase hydrodechlorination of tetrachloromethane has been established. Very small Pt particles (<2 nm) supported on-alumina exhibit low activity and higher selectivity towards C2-dimeric species and methane at the expense of chloroform. A similar, but much less drastic trend, was found for silica-supported Pt catalysts [16].
To the best of our knowledge, the influence of the transition metal precursors and the pretreatment conditions on the catalytic activity of carbon supported nickel catalysts in the aqueous phase hydrodechlorination has never been tested. Therefore, the aim of our study was to investigate the effect of nickel precursor and pretreatment temperature on catalytic behavior of nickel catalysts in the aqueous phase hydrodechlorination of 1,1,2-trichloroethene.
Experimental
Catalysts preparation
Commercial activated carbon Norit CNR 115 with specific surface area 1860 m2/g and pore volume 1.05 cm3/g (supplied by Norit B.V. Company) was used as a starting material in the support synthesis. Norit CNR115 was modified by a two-step procedure. The first step was the heating of raw carbon pellets in argon at 2173 K for 2 h, and the second step was the gasification of the obtained material in the stream of steam-argon at 1129 K, which resulted in mass loss up to 26.54 wt%. The first step of modification led to a considerable carbon graphitization and drastic decrease of the specific surface area (from 1860 to 16 m2/g) and pore volume (from 1.05 to 0.005 cm3/g). After the second step, the specific surface area and pore volume were equal to 1540 m2/g and 0.97 cm3/g, respectively. The obtained ordered carbon material was washed with distilled water, dried in air at 393 K and was used as the support for the synthesis of the nickel catalysts.
Nickel catalysts were prepared by incipient wetness impregnation using active carbon as the support and aqueous solutions of two nickel salts [nickel(II) nitrate and nickel(II) chloride] as the metal precursors. The catalysts prepared from nickel nitrate are marked in the text as Ni(N), while catalysts prepared from nickel chloride are denoted as Ni(Cl). In each case, the overall nickel loading was 2 wt%. The catalyst precursors were impregnated into the support using rotating beaker with simultaneous heating (with a standard 200 W infrared lamp) for 24 h. Before catalytic reactions, the metal precursors were dried in argon flux and then reduced to metal phase in a mixture of 10% H2 in argon flow in two different ways:
-
1.
At low temperature (673 K for 3 h)—catalysts marked as Ni(N)–M and Ni(Cl)–M,
-
2.
At high temperature (from 293 up to 1173 K with the heating rate 10 K/min)—catalysts reduced in this procedure were marked as Ni(N)–H and Ni(Cl)–H.
Catalysts characterization and catalytic tests
Temperature-programmed reduction
Temperature-programmed reduction (TPR) was carried out in a glass flow system equipped with a reactor containing a fritted disk to place the catalyst sample and a Gow-Mac thermal conductivity detector. Catalyst precursors were heated with 10 K/min ramp to 1173 K in the flow of 10% H2/Ar.
X-ray diffraction
X-ray diffraction (XRD) measurements were done with a Rigaku-Denki diffractometer (Japan) equipped with copper lamp Cu Kα and nickel filter. Diffraction profiles were scanned in angles 2θ in the range of 5–95.
TEM measurement
TEM experiments were carried out on the electron microscope Titan G2 60–300 kV (FEI, Japan) equipped with a field emission gun (FEG), monochromator, a three condenser lense system, the objective lens system, image correction (Cs-corrector), a HAADF detector and an EDS spectrometer (energy dispersive X-ray spectroscopy, EDAX, USA) with Si(Li) detector. Microscopic studies of the catalysts were carried out at an accelerating voltage of the electron beam equal to 300 kV. The samples were prepared by their dispersing in pure alcohol using ultrasonic cleaner and putting a drop of this suspension on carbon films on copper grids.
Hydrodechlorination of TCE
The hydrodechlorination reaction of TCE was performed at room temperature (303 K) in a glass reactor equipped with a pH-meter, magnetic stirring bar and temperature controller.
Prior to each catalytic reaction, 350 ml of MiliPore water was added to the reactor and saturated with hydrogen for 30 min. After this time, the hydrogen flow was turned off and then 2.0 µl of TCE was added. After that, the reaction mixture was stirred (1000 rpm) for 30 min to provide homogeneity. Next, 0.1 g of the catalyst was added to the mixture. Reaction samples were taken at 0, 2, 5, 10, 15, 20, 60, 90, 120, 150 min of the reaction. Samples were placed in a headspace system (SHS-40), where they were shaken for time required to obtain state of equilibrium between liquid and headspace. The concentrations of substrate and products were monitored by a gas chromatographic set-up (Bruker 456GC equipped with ECD and FID detectors and Headspace SHS-40).
Temperature-programmed hydrogenation
The catalysts after the hydrodechlorination of TCE in the aqueous phase were investigated by temperature-programmed hydrogenation (TPH) using 20% H2/He flow (25 cm3/min), ramping the temperature at 10 K/min, started from 300 to 800 K. Twelve masses were monitored during the experiment using mass spectrometer MA200 (Dycor-Ametek, Pittsburgh), but particular attention was focused on the evolution of m/z 15 (methane release), m/z 28 (C2Hx release) and m/z 36 (HCl liberation).
Results and discussion
TPR results
Temperature-programmed reduction experiments were carried out to determine the reducibility of nickel in Ni(N)/C and Ni(Cl)/C materials. TPR patterns (Fig. 1) show that the overall reduction of both nickel salts takes place at temperatures lower than 800 K. This allows to assume that both reduction conditions before the catalytic tests (at low and high temperatures) are sufficient to obtain complete reduction of precursors into metallic phases. The TPR pattern of Ni(N) contains one main reduction peak at 553 K and two small peaks with maxima at 634 and 756 K. On the other hand, the TPR pattern of Ni(Cl), shown in Fig. 1, contains two additional positive peaks: one at 650 K and the most prominent peak at 731 K. The broad and asymmetric reduction peak at 731 K is probably associated with the reduction of Ni2+, which is involved in strong interaction with the carbon support, a feature also observed by other groups [17, 18].
These results are in agreement with earlier studies [2], where the carbon support had beneficial effects on the decreasing of the reduction temperature of nickel precursors to its metallic (zerovalent) form.
XRD results
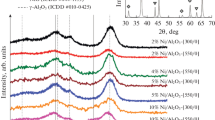
The analysis of diffraction profiles of Ni(N)–H, Ni(Cl)–H, Ni(N)–M and Ni(Cl)–M allows the evaluation of the size of the metal particles for catalysts obtained from different precursors, pretreated under different conditions, both after reduction and after TCE hydrodechlorination (marked with “_TCE” suffix”) (Figs. 2 and 3; Table 1). The size of the metal particles was calculated from the Scherrer equation, based on the Ni (200) reflection. In the case of the Ni(N)–M catalyst, characteristic reflections for Ni were not observed, which suggests the presence of well dispersed Ni nanoparticles with diameters below 3 nm (Fig. 2). The application of the high reduction temperature resulted in the sintering and the formation of larger nickel particles with the average size equal to 18 nm (Table 1). In the case of catalysts prepared with nickel chloride, this phenomenon was not observed. Independently of the reduction conditions, similar particles with the average size of 16 nm were obtained for both catalysts (Fig. 3).
The XRD analysis of the catalysts after reaction with TCE in aqueous phase showed that the reaction conditions do not significantly affect the structure of catalysts reduced at different temperatures (Figs. 2 and 3). Differences in the metal particle size between catalysts before and after reaction were rather slight (Table 1).
In addition, the XRD patterns of all samples contain reflections characteristic for the turbostratic structure of carbon support (peaks at ~43.5° and ~79°) obtained by the two-step modification of Norit CNR115 [19] (Figs. 2, 3).
TEM investigation
TEM micrographs allowed the visualization of the structure of the prepared catalysts. The presence of parallel graphene layers in the carbon material after the preheating at 2173 K and gasification step is obvious (Fig. 4). It was also possible to calculate the average particle sizes from the obtained images and the results are summarized in the Table 1. The TEM images and the calculated average Ni particle size distribution of all reduced catalysts are shown in Fig. 4. The smallest particles sizes (~3 nm) were obtained for the catalyst prepared from nickel nitrate and reduced at the temperature 673 K for 3 h [Ni(N)–M]. This result confirms our observation based on XRD results that the application of nitrate salt of nickel(II) as the metal precursor leads to formation of catalysts with extremely small and well dispersed metal particles. Usually, the application of Ni(NO3)2·6H2O as a metal precursor and active carbon as the support led to formation smaller nickel particles with the average Ni particle size >6 nm than in the case of other salts [15, 20, 21]. Additionally, it is worth noting that the formation of very small nickel particles (~3 nm) by the simple incipient wetness impregnation of the carbon support by nickel nitrate solution is rather unique [22]. As expected, the application of higher reduction temperature leads to the formation of uniformly dispersed but much bigger nickel nanoparticles with an average particle size about 31 nm (Fig. 4).
A different trend can be observed in the results of characterization of the catalyst prepared from nickel chloride [Ni(Cl)]. Independently on the pretreatment conditions (lower or higher temperature), the reduction of nickel precursor in the flow of 10% H2/Ar led to the formation of the metallic catalysts with almost the same average metal particle size: 28–29 nm (Table 1; Fig. 4).
It would appear that Ni2+ ions from nitrate solution deposited on activated carbon are more resistant to sintering while undergoing 3 h reduction at 673 K. However, they are very susceptible to agglomeration caused by exposition to relatively fast heating mode (10 K/min) to the final temperature of 1173 K in the mixture of 10% H2/Ar. On the other hand, the reduction conditions (lower or higher temperature) did not affect the nickel particles size while the catalyst was synthesized from nickel chloride solution.
Differences between the particles size determined from XRD and TEM measurement were observable for all of the catalysts. However, the general trend has been preserved. Based on the literature data [23], we could explain that the average size of nickel-based nanoparticles estimated from XRD data is congruous with that measured by the TEM method only in the case when there are no very small ones among the crystallites.
Catalytic activity
Nickel catalysts prepared from nitrate and chloride precursors, after reduction at two different temperatures, were investigated in the aqueous phase hydrodechlorination of 1,1,2-trichloroethene.
Fig. 5 shows the catalytic results obtained for Ni(Cl)–C. Disparities in the activities between the nickel catalysts reduced at 675 and at 1173 K were observed from the initial time of the hydrodechlorination. The application of Ni(Cl)–H (with average Ni particle size of 29 nm) resulted in the removal of 38% of 1,1,2-TCE till 20 min of reaction, whereas Ni(Cl)–M (with an average particle Ni particle size 28 nm from TEM) removed only 28%. However, the differences in the catalytic activity were reduced after 20 min of the reaction and the discrepancy in conversion after 150 min was equal to 5%, in favor of Ni(Cl)–H.
The analysis of the catalytic results obtained for the Ni(N) catalysts reduced at different temperatures showed surprisingly similar activity for Ni(N)–H (with an average Ni particles size 31 nm) and Ni(N)–M (with an average Ni particles size 3 nm) during the first 20 min of the reaction (Fig. 6). However, the disparity in the conversion after 150 min of reaction was equal to 16% in favor of Ni(N)–H with larger nickel particles size.
The chromatographic analysis of the reaction mixtures showed that TCE hydrodechlorination in the presence of the nickel catalysts leads to the formation of ethane and ethene as the main products. In contrast to the carbon material, which showed only high adsorption capacity of TCE, without the formation of any products (Figs. 5 and 6). Thus, the catalytic behavior of the four Ni/C catalysts depend on the high adsorption affinity of active carbon for low weight polar organic compounds like TCE and the presence of nickel active phase.
A linear approximation of experimental points allowed to calculate the reaction rate constant k using the equation: ln(C/C0) = −kt, where t is time of reaction [s], C0 is the initial TCE concentration and C is the TCE concentration at time t. The initial reaction rates rA were estimated from Eq. (1) [24]:
Here molt0 is the initial moles of 1,1,2-TCE, moltn is the moles of 1,1,2-TCE at analyzed time, tn = 300 s, m is the catalysts mass [g]). The reaction rate constant and initial reaction rates for carbon supported nickel catalysts are summarized in Table 2.
Based on literature data, the hydrodechlorination of TCE should be a pseudo-first order reaction [25]. However, the kinetics of TCE hydrodechlorination for Ni catalysts did not show a straight line characteristic for pseudo-first order reaction. Based on the literature data [26], the reaction kinetics was divided into two parts for each catalyst: before 60 min, with the highest value of the reaction rate constant k obtained for Ni(Cl)–H (Table 2) and from 60 to 150 min of the reaction, with the highest value of reaction rate constant k obtained for Ni(N)–H (Table 2). The decreasing of the reaction rate after 1 h of the TCE hydrodechlorination was observed in the case of all the catalysts (Table 2). Our results are in agreement with earlier studies of Dong et al. [26] and could be explained by the progressive agglomeration of nickel particles under the reaction conditions and/or partial deactivation of nickel active sites by irreversible adsorption of products. The XRD experiments of the used catalysts showed that there are no significant changes in the size of the metal particles after hydrodechlorination reaction. On the other hand, TPH measurements performed for the used catalyst confirmed that deactivation could be related to the irreversible adsorption of products (see Sect. 3.5).
The relationship between metal particles size and the catalytic activity, both for Ni(Cl)/C and for Ni(N)/C indicates that the larger nickel particles (obtained at high reduction temperature) results in higher hydrodechlorination activity in the aqueous phase reaction. This phenomenon is in agreement with earlier studies for noble metals in the hydrodechlorination [27, 28, 29]. This sensitivity is often explained by the higher resistance to deactivation observed for the large metal particles and/or the differences in the hydrogen adsorption mechanisms for the different metal crystallite size. However, it is not always clear. Zhou et al. [29] postulated the volcano type of the relationship between the hydrodechlorination activity and metal particles size. On the other hand, recently published results of the aqueous phase hydrodechlorination of chloroorganic compounds indicate the beneficial role of the small metal particles on overall activity of the metallic catalysts [26, 30, 31]. Our results showed that the aqueous phase hydrodechlorination of 1,1,2-trichloroethene is structurally sensitive, and large nickel particles (~30 nm) are the most active for this reaction under the studied conditions. Although the differences in activity are observable, we expected more spectacular differences in activity of 3 and 31 nm Ni particles.
This means that the synthesis method, the kind of the support and the metal(s) particles size may greatly influence on the catalytic activity in different reaction conditions.
In summary, the catalytic activity of mesoporous carbon supported nickel in the aqueous phase hydrodechlorination of 1,1,2-trichloroethene increases with the increasing of metal particles size, especially in the case of nickel catalyst synthesized from nitrate solution. Ni/C catalysts synthesized from nickel nitrate are more sensitive to the pretreatment conditions than the catalysts obtained from nickel chloride. The kind of salt does not matter for the activity of the catalysts reduced at high temperature regime.
TPH results
Temperature-programmed hydrogenation (TPH) measurements were carried out for spent Ni(Cl)–M catalyst [Ni(Cl)–M_TCE]. Fig. 7 shows that during the aqueous-phase hydrodechlorination of TCE, mainly carbon containing species were deposited on the catalyst surface (m/z 15 and m/z 28, methane and C2Hx release, respectively). These deposits were strongly bounded with Ni(Cl)–M_TCE surface and desorbed at about 700 K. We assume that they probably blocked active metal sites, what in consequence could lead to deactivation of the catalysts. The deposits containing chlorine (m/z 36, HCl liberation) were not observed during the aqueous-phase hydrodechlorination. Our results are in agreement with earlier data concerning similar results obtained for Ni/CST−A and Ni–Pd/C catalysts after the aqueous phase reaction with TCE [31].
Conclusions
The conducted experiments and the analysis of the obtained data allowed us to get some conclusions about catalytic activity of Ni/C catalysts. Mesoporous carbon supported nickel proved to be the active catalyst in the aqueous phase hydrodechlorination of TCE. The catalytic properties of nickel samples depended on the kind of nickel precursor used for Ni/C synthesis and the pretreatment conditions. The highest catalytic activity, both for Ni(N)/C and Ni(Cl)/C catalyst, was observed for catalysts with larger Ni particles size.
References
Huang B, Lei C, Wei C, Zeng G (2014) Environ Inter 71:118–138
Wei J, Qian Y, Liu W, Wang L, Ge Y, Zhang J, Yu J, Ma X (2014) J Environ Sci 26:1162–1170
Śrębowata A, Kamińska II, Giziński D, Wideł D, Oszczudłowski J (2015) Catal Today 251:60–65
Zhang M, Bacik DB, Roberts CB, Zhao D (2013) Water Res 47:3706–3715
Barrabes N, Fottinger K, Dafinov A, Medina F, Rupprechter G, Llorca J, Suaeiras JE (2009) Appl Catal B 87:84–91
Ordonez S, Diaz E, Diez FV, Sastre H (2007) React Kinet Catal Lett 90:101–106
Kim P, Kim Y, Kang T, Song IK, Yi J (2007) Catal Surv Asia 11:49–58
Śrębowata A, Baran R, Lisovytskiy D, Kamińska II, Dźwigaj S (2014) Catal Commun 57:107–110
Kamińska II, Śrębowata A (2015) Chem Intermed 41:9267–9280
Meshesha BT, Barabes N, Fottinger K, Chimentao RJ, Llorca J, Medina F, Rupprechter G, Sueiras JE (2012) Appl Catal B 117–118:236–245
Radovic LR, Moreno-Castilla C, Rivera-Utrilla J (2000) In: Radovic LR (ed) Chemistry and physics of carbon, vol 27. Marcel Dekker, New York
Gil A, de la Puente G, Grange P (1997) Microporous Mater 12:51–61
Martin-Martinez M, Gomez-Sainero LM, Palomar J, Omar S, Rodriguez JJ (2016) Catal Lett 146:2614–2621
Kim P, Joo JB, Kim W, Song IK, Yi J (2006) J Mol Catal A 256:178–183
Śrębowata A, Juszczyk W, Kaszkur Z, Sobczak JW, Kępiński L, Karpiński Z (2007) Appl Catal A 319:181–192
Bonarowska M, Kaszkur Z, Kępiński L, Karpiński Z (2010) Appl Catal B 99:248–256
Nivea MA, Villaverde MM, Monzon A, Garetto T, Marchi A (2014) J. Chem Eng J 235:158–166
Wang W, Chu W, Wang N, Yang W, Jiang C (2016) Inter J Hydrog Energy 41:967–975
Bonarowska M, Raróg-Pilecka W, Karpiński Z (2011) Catal Today 169:223–231
Wang J, Fan G, Li F (2012) RSC Adv 2:9976–9985
Wu W, Xu J (2004) Catal Commun 5:591–595
Fidalgo B, Zubizarreta L, Bermúdez JM, Arenillas A, Menéndez JA (2010) Fuel Process Technol 91:765–769
Słowik G, Gawryszuk-Rżysko A, Greluk M, Machocki A (2016) Catal Lett 146:2173–2184
Cobo M, Becerra J, Castelblanco M, Cifuentes B, Conesa JA (2015) J Environ Manag 158:1–10
Ordonez S, Vivas BP, Diez FV (2010) Appl Catal B 95:288–296
Dong Z, Le X, Dong Ch, Zhang W, Li X, Ma J (2015) Appl Catal B 162:372–380
Salih HH, Sorial GA, Patterson CL, Sinha R, Krishnan ER (2012) Water Air Soil Pollut 223:2837–2847
Diaz E, Faba L, Ordonez S (2011) Appl Catal B 104:415–417
Janiak T, Okal J (2009) Appl Catal B 92:384–392
Zhou J, Wu K, Wang W, Xu W, Wan Z, Wan H, Zheng S (2014) Appl Catal A 470:336–343
Śrębowata A, Tarach K, Girman V, Góra-Marek K (2016) Appl Catal B 181:550–560
Śrębowata A, Kamińska I (2015) Recycl Catal 2:17–22
Acknowledgements
This work was partially founded by National Science Centre in Poland (Research Project SONATA based on decision DEC-2011/03/D/ST5/05516). We would like to thank Wioletta Raróg-Pilecka from Technical University in Warsaw for carbon synthesis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kowalewski, E., Kamińska, I.I., Słowik, G. et al. Effect of metal precursor and pretreatment conditions on the catalytic activity of Ni/C in the aqueous phase hydrodechlorination of 1,1,2-trichloroethene. Reac Kinet Mech Cat 121, 3–16 (2017). https://doi.org/10.1007/s11144-017-1148-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-017-1148-4