Abstract
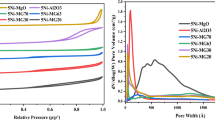
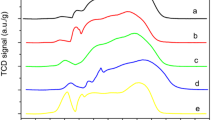
The sintering behavior of a co-precipitated Ni/Al2O3 methanation catalyst is studied by investigating the effect of treating time, temperature and atmosphere. Fresh and sintered samples are characterized by N2 physisorption, H2 chemisorption, temperature programmed reduction, X-ray diffraction and transmission electron microscopy. A reduction both in total and nickel surface area has been observed, the extent depending on the experimental conditions. Sintering of the studied catalyst, reflected by a significant decrease of nickel surface area, is a combined effect of primary encapsulation of metallic nickel due to the collapse of the support structure and sporadic agglomeration of nickel crystallites. The formation of a Ni2+ doped alumina phase, induced by steam ambience, further accelerates loss of surface nickel atoms. It is found that the sintering rate obeys a simple power law expression, with the apparent activation energy value of 118 kJ/mol. The sintered methanation catalyst suffers considerable decay of CO hydrogenation activity in a simulated industrial atmosphere, which suggests that extraordinarily high temperatures should be avoided as much as possible in the practical operation.






Similar content being viewed by others
References
Mills GA, Steffgen FW (1973) Catalytic methanation. Catal Rev 8:159–210
Rostrup-Nielsen JR, Pedersen K, Sehested J (2007) High temperature methanation Sintering and structure sensitivity. Appl Catal A Gen 330:134–138
Ma SL, Tan YS, Han YZ (2011) Methanation of syngas over coral reef-like Ni/Al2O3 catalysts. J Nat Gas Chem 20:435–440
Wynblatt P, Gjostein NA (1975) Supported metal crystallites. Prog Solid State Chem 9:21–58
Wanke SE, Flynn PC (1975) The sintering of supported metal catalysts. Catal Rev 12:93–135
Bartholomew CH (2001) Mechanisms of catalyst deactivation. Appl Catal A Gen 212:17–60
Sehested J (2003) Sintering of nickel steam-reforming catalysts. J Catal 217:417–426
Gabrovska M, Edreva-Kardjieva R, Crişan D, Tzvetkov P, Shopska M, Shtereva I (2012) Ni–Al layered double hydroxides as catalyst precursors for CO2 removal by methanation. Reac Kinet Mech Cat 105:79–99
Hughes R (1984) Deactivation of catalysts. Academic Press, London
Saletore DA, Thomson WJ (1977) Methanation reaction rates for recycle reactor compositions. Ind Eng Chem Proc Des Dev 16:70–75
Pedersen K, Skov A, Rostrup-Nielsen JR (1980) Catalytic aspects of high temperature methanation. ACS Preprints Div Fuel Chem 25:89–100
Arabczyk W, Jasińska I, Lendzion-Bieluń Z (2011) Kinetics studies of recrystallization process of metallic catalysts for ammonia synthesis. Catal Today 169:93–96
Agnelli M, Kolb M, Mirodatos C (1994) CO hydrogenation on a nickel catalyst. J Catal 148:9–21
Lif J, Odenbrand I, Skoglundh M (2007) Sintering of alumina-supported nickel particles under amination conditions: support effects. Appl Catal A Gen 317:62–69
Teixeira ACSC, Giudici R (1999) Deactivation of steam reforming catalysts by sintering: experiments and simulation. Chem Eng Sci 54:3609–3618
Vogelaar BM, van Langeveld AD, Kooyman PJ, Lok CM, Bonné RLC, Moulijn JA (2011) Stability of metal nanoparticles formed during reduction of alumina supported nickel and cobalt catalysts. Catal Today 163:20–26
Campanati M, Fornasari G, Vaccari A (2003) Fundamentals in the preparation of heterogeneous catalysts. Catal Today 77:299–314
Li GH, Hu LJ, Hill JM (2006) Comparison of reducibility and stability of alumina-supported Ni catalysts prepared by impregnation and co-precipitation. Appl Catal A Gen 301:16–24
Williams A, Butler GA, Hammonds J (1972) Sintering of nickel–alumina catalysts. J Catal 24:352–355
Sehested J, Gelten JAP, Helveg S (2006) Sintering of nickel catalysts: effects of time, atmosphere, temperature, nickel–carrier interactions and dopants. Appl Catal A Gen 309:237–246
Sehested J, Gelten JAP, Remediakis IN, Bengaard H, Nørskov JK (2004) Sintering of nickel steam-reforming catalysts: effects of temperature and steam and hydrogen pressures. J Catal 223:432–443
Sadeqzadeh M, Hong JP, Fongarland P, Curulla-Ferré D, Luck F, Bousquet J, Schweich D, Khodakov AY (2012) Mechanistic modeling of cobalt based catalyst sintering in a fixed bed reactor under different conditions of Fischer-Tropsch synthesis. Ind Eng Chem Res 51:11955–11964
Clause O, Rebours B, Merlen E, Trifiró F, Vaccari A (1992) Preparation and characterization of nickel–aluminum mixed oxides obtained by thermal decomposition of hydrotalcite-type precursors. J Catal 133:231–246
Scheffer B, Molhoek P, Moulijn JA (1989) Temperature-programmed reduction of NiOWO3/Al2O3 hydrodesulphurization catalysts. Appl Catal 46:11–30
Acknowledgments
We acknowledge the National High Technology Research and Development Program of China (863 Program, 2009AA050901) and the National Natural Science Foundation of China (21276250) for funding this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bai, X., Wang, S., Sun, T. et al. The sintering of Ni/Al2O3 methanation catalyst for substitute natural gas production. Reac Kinet Mech Cat 112, 437–451 (2014). https://doi.org/10.1007/s11144-014-0700-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-014-0700-8