Abstract
Purpose
To report the results of a randomized controlled trial using an electronic monitoring device (EM) plus a motivational interviewing (MI) intervention to enhance adherence to disease-modifying therapies (DMT) in pediatric MS.
Methods
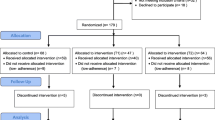
Fifty-two youth with MS (16.03 ± 2.2 years) were randomized to receive either MI (n = 25) (target intervention) or a MS medication video (n = 27) (attention control). Primary endpoint was change in adherence. Secondary outcomes included changes in quality of life, well-being and self-efficacy. Random effects modeling and Cohen’s effect size computation evaluated intervention impact.
Results
Longitudinal random effect models revealed that the MI group decreased their EM adherence (GroupxTime interaction = −0.19), while increasing frequency of parental DMT reminder (26.01)/administration (11.69). We found decreased EM use in the MI group at 6 months (Cohen’s d = −0.61), but increased pharmacy refill adherence (d = 0.23). Parental reminders about medication increased in MI subjects vs controls (d = 0.59 at 3 months; d = 0.70 at 6 months). We found increases in self-reported adherence (d = 0.21) at 3 but not 6 months, fewer barriers to adherence at three (d = −0.58) and six months (d = −0.31), better physical (d = 0.23 at 3 months; d = 0.45 at 6 months), emotional (d = 0.25 at 3 months) and self-efficacy function (d = 0.55 at 3 months; 0.48 at 6 months), but worse well-being, including self-acceptance (d = −0.53 at 6 months) and environmental mastery (d = −0.42 at 3 and 6 months) in intervention as compared to control patients.
Conclusions
Participants receiving MI + EM experienced worsening on objective measures of adherence and increased parental involvement, but improved on some self- and parent-reported measures. MI participants reported improvements in quality of life and self-efficacy, but worsened well-being.



Similar content being viewed by others
Change history
23 December 2017
The clinicaltrials.gov identifying number for the article titled “Impact of an electronic monitoring device and behavioral feedback on adherence to multiple sclerosis therapies in youth: results of a randomized trial” is NCT02234713 (https://clinicaltrials.gov/ct2/show/NCT02234713).
Abbreviations
- DMT:
-
Disease-modifying therapy
- EM:
-
Electronic monitoring
- MI:
-
Motivational interviewing
- MS:
-
Multiple sclerosis
- MSSE:
-
MS Self-Efficacy Scale
- MSTAQ:
-
Multiple Sclerosis Treatment Adherence Questionnaire
- PedsQL:
-
Pediatric Quality of Life Inventory
- SD:
-
Standard deviation
References
Yeh, E. A., Waubant, E., Krupp, L. B., Ness, J., Chitnis, T., Kuntz, N., et al. (2011). Multiple sclerosis therapies in pediatric patients with refractory multiple sclerosis. Archives of Neurology, 68(4), 437–444. doi:10.1001/archneurol.2010.325.
Thannhauser, J. E., Mah, J. K., & Metz, L. M. (2009). Adherence of adolescents to multiple sclerosis disease-modifying therapy. Pediatric Neurology, 41(2), 119–123. doi:10.1016/j.pediatrneurol.2009.03.004.
Giovannoni, G., Southam, E., & Waubant, E. (2012). Systematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: Tolerability and adherence. Multiple Sclerosis Journal, 18(7), 932–946.
Kahana, S., Drotar, D., & Frazier, T. (2008). Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. Journal of Pediatric Psychology, 33(6), 590–611. doi:10.1093/jpepsy/jsm128.
Kahana, S., Drotar, D., & Frazier, T. (2008). Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. Journal of Pediatric Psychology, 33(6), 590–611.
Graves, M. M., Roberts, M. C., Rapoff, M., & Boyer, A. (2010). The efficacy of adherence interventions for chronically ill children: A meta-analytic review. Journal of Pediatric Psychology, 35(4), 368–382.
Britt, E., Hudson, S. M., & Blampied, N. M. (2004). Motivational interviewing in health settings: A review. Patient Education and Counseling, 53(2), 147–155. doi:10.1016/S0738-3991(03)00141-1.
Sabin, L. L., DeSilva, M. B., Hamer, D. H., Xu, K., Zhang, J., Li, T., et al. (2010). Using electronic drug monitor feedback to improve adherence to antiretroviral therapy among HIV-positive patients in China. AIDS and Behavior, 14(3), 580–589. doi:10.1007/s10461-009-9615-1.
Ruppar, T. M. (2010). Randomized pilot study of a behavioral feedback intervention to improve medication adherence in older adults with hypertension. Journal of Cardiovascular Nursing, 25(6), 470–479. doi:10.1097/JCN.0b013e3181d5f9c5.
Bartlett, S. J., Lukk, P., Butz, A., Lampros-Klein, F., & Rand, C. S. (2002). Enhancing medication adherence among inner-city children with asthma: Results from pilot studies. Journal of Asthma, 39(1), 47–54.
Burgess, S. W., Sly, P. D., & Devadason, S. G. (2010). Providing feedback on adherence increases use of preventive medication by asthmatic children. Journal of Asthma, 47(2), 198–201. doi:10.3109/02770900903483840.
Herzer, M., Ramey, C., Rohan, J., & Cortina, S. (2011). Incorporating electronic monitoring feedback into clinical care: A novel and promising adherence promotion approach. Clinical child psychology and psychiatry. doi:10.1177/1359104511421103.
Krupp, L. B., Banwell, B., & Tenembaum, S. (2007). Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology, 68(16 Suppl 2), S7–12. doi:10.1212/01.wnl.0000259422.44235.a8.
Polman, C. H., Reingold, S. C., Banwell, B., Clanet, M., Cohen, J. A., Filippi, M., et al. (2011). Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology, 69(2), 292–302. doi:10.1002/ana.22366.
Moyers, T. B., Manuel, J. K., & Ernst, D. (2014). Motivational Interviewing Treatment Integrity Coding Manual 4.1.
Graves, M. M., Roberts, M. C., Rapoff, M., & Boyer, A. (2010). The efficacy of adherence interventions for chronically ill children: A meta-analytic review. Journal of Pediatric Psychology, 35(4), 368–382. doi:10.1093/jpepsy/jsp072.
Morisky, D. E., Ang, A., Krousel-Wood, M., & Ward, H. J. (2008). Predictive validity of a medication adherence measure in an outpatient setting. The Journal of Clinical Hypertension, 10(5), 348–354.
Wicks, P., Massagli, M., Kulkarni, A., & Dastani, H. (2011). Use of an online community to develop patient-reported outcome instruments: the Multiple Sclerosis Treatment Adherence Questionnaire (MS-TAQ). Journal of medical Internet research, 13(1), e12.
McKay, K. A., Tremlett, H., Patten, S. B., Fisk, J. D., Evans, C., Fiest, K., et al. (2016). Determinants of non-adherence to disease-modifying therapies in multiple sclerosis: A cross-Canada prospective study. Multiple Sclerosis Journal, 23(4), 588–596. doi:10.1177/1352458516657440.
Varni, J. W., Seid, M., & Kurtin, P. S. (2001). PedsQL™ 4.0: Reliability and validity of the Pediatric Quality of Life Inventory™ Version 4.0 Generic Core Scales in healthy and patient populations. Medical Care, 39(8), 800–812.
Benedict, R. H., Cox, D., Thompson, L. L., Foley, F., Weinstock-Guttman, B., & Munschauer, F. (2004). Reliable screening for neuropsychological impairment in multiple sclerosis. Multiple Sclerosis Journal, 10(6), 675–678.
O’Brien, A., Gaudino-Goering, E., Shawaryn, M., Komaroff, E., Moore, N. B., & DeLuca, J. (2007). Relationship of the Multiple Sclerosis Neuropsychological Questionnaire (MSNQ) to functional, emotional, and neuropsychological outcomes. Archives of Clinical Neuropsychology, 22(8), 933–948.
Schwartz, C. E., Coulthard-Morris, L., Zeng, Q., & Retzlaff, P. (1996). Measuring self-efficacy in people with multiple sclerosis: a validation study. Archives of Physical Medicine and Rehabilitation, 77(4), 394–398. doi:10.1016/S0003-9993(96)90091-X.
Ryff, C. D. (1989). Happiness is everything, or is it? explorations on the meaning of psychological well-being. Journal of Personality and Social Psychology, 57, 1069–1081.
Schwartz, C. E., Keyl, P., Bode, R., & Marcum, J. (2009). Helping others shows differential benefits on health and well-being for male and female teens. Journal of Happiness Studies, 10(4), 431–448.
Hohol, M. J., Orav, E. J., & Weiner, H. L. (1995). Disease steps in multiple sclerosis: A simple approach to evaluate disease progression. Neurology, 45, 251–255.
Schwartz, C. E., Vollmer, T., & Lee, H. (1999). Reliability and validity of two self-report measures of impairment and disability for MS. North American Research Consortium on Multiple Sclerosis Outcomes Study Group. Neurology, 52(1), 63–70.
Cohen, J. (1992). A power primer. Psychological Bulletin, 112, 155–159.
StataCorp. (2016). Stata statistical software: release 14. College Station: StataCorp LP.
Nazareth, M., Richards, J., Javalkar, K., Haberman, C., Zhong, Y., Rak, E., et al. (2016). Relating health locus of control to health care use, adherence, and transition readiness among youths with chronic conditions, North Carolina, 2015. Preventing chronic disease, 13, E93. doi:10.5888/pcd13.160046.
Klassen, A. F., Miller, A., & Fine, S. (2006). Agreement between parent and child report of quality of life in children with attention-deficit/hyperactivity disorder. Child: Care, Health and Development, 32(4), 397–406. doi:10.1111/j.1365-2214.2006.00609.x.
Jozefiak, T., Larsson, B., Wichstrom, L., Mattejat, F., & Ravens-Sieberer, U. (2008). Quality of Life as reported by school children and their parents: A cross-sectional survey. Health and Quality of Life Outcomes, 6, 34. doi:10.1186/1477-7525-6-34.
Meyer, T. J., & Mark, M. M. (1995). Effects of psychosocial interventions with adult cancer patients: a meta-analysis of randomized experiments. Health Psychology, 14(2), 101–108.
Grossman, P., Niemann, L., Schmidt, S., & Walach, H. (2004). Mindfulness-based stress reduction and health benefits A meta-analysis. Journal of psychosomatic research, 57(1), 35–43. doi:10.1016/S0022-3999(03)00573-7.
Norman, G. R., Sloan, J. A., & Wyrwich, K. W. (2003). Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Medical Care, 41(5), 582–592. doi:10.1097/01.MLR.0000062554.74615.4C.
Acknowledgements
We are grateful for the involvement of the youth with MS and their parents, as well as to all the investigators and their institutions involved, without whom this study would not have been possible.
Funding
This work was funded by the National Multiple Sclerosis Society (HC 0148).
Pediatric MS Adherence Study Group
Gregory Aaen, Gulay Alper, Brenda L. Banwell, Charlene Belsole, Tara Berenbaum, Petra Breiner, Susana Camposano, Hardeep Chohan, Carolynn Darrell, Sarah Dowdy, Kim Edwards, Mark Gorman, Jennifer Graves, La June Grayson, Stephanie A. Grover, Tiffany Haig, Sabrina Hamer, Janace Hart, Kawonas Jenkins, Amy Lavery, Geraldine Liu, Timothy Lotze, Jean K. Mah, Rory Mahabir, Soe Mar, Lauren Mednick, Elva R. Mendoza, Manikum Moodley, Jayne Ness, Austin Noguera, Maya Obadia, Marvin Petty, Sarah Planchon Pope, Daniela Pohl, Mariam Pontifes, Victoria E. Powell, Elizabeth Quon, Mary Rensel, Jennifer Resto, Ian Rossman, Melissa Rundquist, Karla Sanchez, Teri Schreiner, Carolyn E. Schwartz, Ruth Slater, Maleka Smith, Jaime Sorum, Alexander Stein, Marija Stosic, Jan-Mendelt Tillema, Sunita Venkateswaran, Jennifer Vincent, Amy Waldman, Emmanuelle Waubant and E. Ann Yeh.
Availability of data and supporting materials
Supporting documentation for our findings is provided in manuscript data and figures and tables. Scientists wishing to gain access to our data may contact the first author (EAY), who will consider such requests on a case-by-case basis, subject to the scientific rigor of the proposed research question.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors (SG, VEP, GA, KE, MG, TEM, JM, LM, JN, MO, RS, EW and CES) have no relevant conflicts of interest to disclose. EAY and CES wrote the first draft of the manuscript and neither received an honorarium, grant, or other form of payment to do so. BLB serves as a consultant to Novartis for the purposes of a clinical trial and as an unpaid advisor to Biogen, Teva neuroscience and Sanofi. She is also a chief editor for Multiple Sclerosis and Related Disorders and is on the editorial board for Neurology. JG has received grant funding from the Race to Erase MS, Biogen and Genentech. AW has received grant funding from the National Institutes of Health (USA) and Biogen Idec. EAY receives research funding from NMSS, CMSC, OIRM, SCN, CBMH Chase an Idea, SickKids Foundation, Rare Diseases Foundation, MS Scientific Foundation (Canada), McLaughlin Centre, Mario Batalli Foundation. She performs relapse adjudication for ACI, has received unrestricted funding for a symposium from the Guthy Jackson Charitable Foundation and Teva and has served on a Scientific Advisory Board for Neurotoxicity with Juno Pharmaceuticals.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants and their parent or legal guardian included in this study.
Study Sites
We are also grateful for the hard work and dedication of the investigators and study teams at each site (Table 7).
Rights and permissions
About this article
Cite this article
Yeh, E., Grover, S.A., Powell, V.E. et al. Impact of an electronic monitoring device and behavioral feedback on adherence to multiple sclerosis therapies in youth: results of a randomized trial. Qual Life Res 26, 2333–2349 (2017). https://doi.org/10.1007/s11136-017-1571-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-017-1571-z