Abstract
Purpose
Health-related quality of life (HRQOL) is important in spina bifida (SB) management. No clinically useful, comprehensive instrument incorporating bladder/bowel domains exists. We aimed to develop and validate a self-reported QUAlity of Life Assessment in Spina bifida for Adults (QUALAS-A).
Methods
We drafted the 53-question pilot instrument using a comprehensive item generation/refinement process. It was administered to an international convenience sample of adults with SB and controls recruited online via social media and in person at outpatient SB clinics (January 2013–September 2014). Final questions were determined by: clinical relevance, high factor loadings and domain psychometrics in an Internal Validation Sample randomly selected from United States participants (n = 250). External validity was evaluated in United States and International External Validation Samples (n = 165 and n = 117, respectively). Adults with SB completed the validated general WHOQOL-BREF and International Consultation on Incontinence Questionnaire (ICIQ).
Results
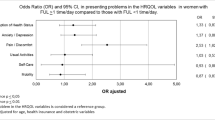
Mean age of 532 participants was 32 years (32.7 % males, 85.0 % Caucasian), similar to 116 controls (p ≥ 0.08). There were 474 online and 58 clinical participants (61.1 % eligible). Face validity and content validity of the 3-domain, 15-question QUALAS-A were established by patients, families and experts. Internal consistency and test–retest reliability were high for all domains (Cronbach’s alpha ≥ 0.70, ICC ≥ 0.77). Correlations between QUALAS-A and WHOQOL-BREF were low (r ≤ 0.60), except for high correlations with Health and Relationships domain (0.63 ≤ r ≤ 0.71). Bladder and Bowel domain had a high correlation with ICIQ (r = −0.70). QUALAS-A scores were lower among adults with SB than without (p < 0.0001). QUALAS-A had good statistical properties in both External Validation Samples (Cronbach’s alpha 0.68–0.77).
Conclusions
QUALAS-A is a short, valid HRQOL tool for adults with SB.


Similar content being viewed by others
Abbreviations
- HRQOL:
-
Health-related quality of life
- QOL:
-
Quality of life
- SB:
-
Spina bifida
- QUALAS-A:
-
QUAlity of Life Assessment in Spina bifida for Adults
- WHOQOL-BREF:
-
World Health Organization Quality of Life instrument
- SD:
-
Standard deviation
References
Boulet, S. L., Yang, Q., Mai, C., Kirby, R. S., Collins, J. S., Robbins, J. M., et al. (2008). Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Research Part A: Clinical and Molecular Teratology, 82(7), 527–532.
Vajda, P., Kispal, Z., Lenart, I., Farkas, A., Vastyan, A. M., & Pinter, A. B. (2009). Quality of life: Urinary bladder augmentation or substitution in children. Pediatric Surgery International, 25(2), 195–201.
Salter, M. J. (1992). What are the differences in body image between patients with a conventional stoma compared with those who have had a conventional stoma followed by a continent pouch? Journal of Advanced Nursing, 17(7), 841–848.
Ellsworth, P. I., Webb, H. W., Crump, J. M., Barraza, M. A., Stevens, P. S., & Mesrobian, H. G. (1996). The Malone antegrade colonic enema enhances the quality of life in children undergoing urological incontinence procedures. Journal of Urology, 155(4), 1416–1418.
Sawin, K. J., & Bellin, M. H. (2010). Quality of life in individuals with spina bifida: A research update. Developmental Disabilities Research Reviews, 16(1), 47–59.
Skevington, S. M., Lotfy, M., O’Connell, K. A., & WHOQOL Group. (2004). The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A report from the WHOQOL group. Quality of Life Research, 13(2), 299–310.
Ware, J. E., Jr., Kosinski, M., Bjorner, J. B., Turner-Bowker, D. M., Gandek, B., & Maruish, M. E. (2007). User’s manual for the SF-36v2TM Health Survey (2nd ed.). Lincoln: RI QualityMetric Incorporated.
Waters, E., Davis, E., Ronen, G. M., Rosenbaum, P., Livingston, M., & Saigal, S. (2009). Quality of life instruments for children and adolescents with neurodisabilities: How to choose the appropriate instrument. Developmental Medicine and Child Neurology, 51(8), 660–669.
Parekh, A. D., Trusler, L. A., Pietsch, J. B., Byrne, D. W., DeMarco, R. T., Pope, J. C. T., et al. (2006). Prospective, longitudinal evaluation of health related quality of life in the pediatric spina bifida population undergoing reconstructive urological surgery. Journal of Urology, 176(4 Pt 2), 1878–1882.
Lemelle, J. L., Guillemin, F., Aubert, D., Guys, J. M., Lottmann, H., Lortat-Jacob, S., et al. (2006). Quality of life and continence in patients with spina bifida. Quality of Life Research, 15(9), 1481–1492.
Patrick, D. L., Burke, L. B., Powers, J. H., Scott, J. A., Rock, E. P., Dawisha, S., et al. (2007). Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health, 10(Suppl 2), S125–137.
Kulkarni, A. V., Rabin, D., & Drake, J. M. (2004). An instrument to measure the health status in children with hydrocephalus: The Hydrocephalus Outcome Questionnaire. Journal of Neurosurgery, 101(2 Suppl), 134–140.
Parkin, P. C., Kirpalani, H. M., Rosenbaum, P. L., Fehlings, D. L., Van Nie, A., Willan, A. R., et al. (1997). Development of a health-related quality of life instrument for use in children with spina bifida. Quality of Life Research, 6(2), 123–132.
Sawicki, G. S., Lukens-Bull, K., Yin, X., Demars, N., Huang, I. C., Livingood, W., et al. (2011). Measuring the transition readiness of youth with special healthcare needs: Validation of the TRAQ—Transition Readiness Assessment Questionnaire. Journal of Pediatric Psychology, 36(2), 160–171.
Kincaid, J. P., Fishburne, L. R. P, Jr, Rogers, R. L., & Chissom, B. S. (1975). Derivation of new readability formulas (Automated Readability Index, Fog Count and Flesch Reading Ease Formula) for Navy enlisted personnel. Research Branch Report, 8–75, 1–48.
Varni, J. W., Seid, M., & Rode, C. A. (1999). The PedsQL: Measurement model for the pediatric quality of life inventory. Medical Care, 37(2), 126–139.
Topolski, T. D., Patrick, D. L., Edwards, T. C., Huebner, C. E., Connell, F. A., & Mount, K. K. (2001). Quality of life and health-risk behaviors among adolescents. Journal of Adolescent Health, 29(6), 426–435.
Ravens-Sieberer, U., Auquier, P., Erhart, M., Gosch, A., Rajmil, L., Bruil, J., et al. (2007). The KIDSCREEN-27 quality of life measure for children and adolescents: Psychometric results from a cross-cultural survey in 13 European countries. Quality of Life Research, 16(8), 1347–1356.
Avery, K., Donovan, J., Peters, T. J., Shaw, C., Gotoh, M., & Abrams, P. (2004). ICIQ: A brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourology and Urodynamics, 23(4), 322–330.
Bryant, F. B., & Yarnold, P. R. (1995). Principal components analysis and exploratory and confirmatory factor analysis. In L. G. Grimm & R. R. Yarnold (Eds.), Reading and understanding multivariale statistics (pp. 99–136). Washington, DC: American Psychological Association.
Gorsuch, R. L. (1983). Factor analysis (2nd ed.). Hillsdale, NJ: L. Erlbaum Associates.
Joliffe, I. T., & Morgan, B. J. (1992). Principal component analysis and exploratory factor analysis. Statistical Methods in Medical Research, 1(1), 69–95.
Streiner, D. L. (1994). Figuring out factors: The use and misuse of factor analysis. Canadian Journal of Psychiatry, 39(3), 135–140.
Streiner, D. L., & Norman, G. R. (2003). Health measurement scales: A practical guide to their development and use (3rd ed.). Oxford: Oxford University Press.
Lu, L. N. S. (2007). Reliability analysis: Calculate and compare intra-class correlation coefficients (ICC) in SAS. In Proceedings of NESUG (NorthEast SAS Users Group).
Cohen, J. (1992). A power primer. Psychol Bull, 112(1), 155–159.
Hays, R. D., Farivar, S. S., & Liu, H. (2005). Approaches and recommendations for estimating minimally important differences for health-related quality of life measures. COPD, 2(1), 63–67.
Norman, G. R., Sloan, J. A., & Wyrwich, K. W. (2004). The truly remarkable universality of half a standard deviation: confirmation through another look. Expert Review of Pharmacoeconomics & Outcomes Research, 4(5), 581–585.
Chassany, O., Sagnier, P., Marquis, P., Fullerton, S., Aaronson, N., & Group, F. t. E. R. I. o. Q. o. L. A. (2002). Patient-reported outcomes: The Example of Health-Related Quality of Life—A European Guidance Document for the Improved Integration of Health-Related Quality of Life Assessment in the Drug Regulatory Process. Therapeutic Innovation & Regulatory Science, 36, 209–238. doi:10.1177/009286150203600127.
Wyrwich, K. W., Bullinger, M., Aaronson, N., Hays, R. D., Patrick, D. L., Symonds, T., & Clinical Significance Consensus Meeting. (2005). Estimating clinically significant differences in quality of life outcomes. Quality of Life Research, 14(2), 285–295.
Bureau, U. S. C. (2011). Population estimates, Vintage 2011: National Tables (Annual Population Estimates).
Sawyer, S. M., & Roberts, K. V. (1999). Sexual and reproductive health in young people with spina bifida. Developmental Medicine and Child Neurology, 41(10), 671–675.
Joyner, B. D., McLorie, G. A., & Khoury, A. E. (1998). Sexuality and reproductive issues in children with myelomeningocele. European Journal of Pediatric Surgery, 8(1), 29–34.
Nanigian, D. K., Nguyen, T., Tanaka, S. T., Cambio, A., DiGrande, A., & Kurzrock, E. A. (2008). Development and validation of the fecal incontinence and constipation quality of life measure in children with spina bifida. Journal of Urology, 180(Suppl 4), 1770–1773; discussion 1773.
Sawin, K. J., Brei, T. J., Buran, C. F., & Fastenau, P. S. (2002). Factors associated with quality of life in adolescents with spina bifida. J Holist Nurs, 20(3), 279–304.
De Civita, M., Regier, D., Alamgir, A. H., Anis, A. H., Fitzgerald, M. J., & Marra, C. A. (2005). Evaluating health-related quality-of-life studies in paediatric populations: Some conceptual, methodological and developmental considerations and recent applications. Pharmacoeconomics, 23(7), 659–685.
Wyrwich, K. W., & Wolinsky, F. D. (2000). Identifying meaningful intra-individual change standards for health-related quality of life measures. Journal of Evaluation in Clinical Practice, 6(1), 39–49.
Acknowledgments
We would like to thank the hundreds of individuals with spina bifida and their parents and caregivers participating in this study. We also want to thank the following individuals and groups for their help: Concept and Content Consultant: Kathleen J. Sawin, PhD CPNP-PC FAAN (College of Nursing, University of Wisconsin-Milwaukee/Children’s Hospital of Wisconsin); Content Consultants: Pediatric Urology: John Paul Capolicchio, MD (Montreal Children’s Hospital, McGill University), Mohamed Elsherbini, MD (Montreal Children’s Hospital, McGill University), Walid Farhat, MD (Hospital for Sick Children, University of Toronto), Richard Grady, MD (Seattle Children’s Hospital, University of Washington), Roman Jednak, MD (Montreal Children’s Hospital, McGill University), David B. Joseph, MD (University of Alabama at Birmingham), Alison C. Keenan, MD (Riley Hospital for Children, Indiana University Health), Martin A. Koyle, MD (Hospital for Sick Children, University of Toronto), Andrew L. MacNeilly, MD (British Columbia Children’s Hospital, University of British Columbia), JL Pippi Salle, MD (Hospital for Sick Children, University of Toronto), Melissa A. Young, CPNP (Riley Hospital for Children, Indiana University Health). Pediatrics: Timothy J. Brei, MD (Seattle Children’s Hospital, University of Washington), Joseph O’Neil, MD (Riley Hospital for Children, Indiana University Health). Clinical Pediatric Psychology: Khush Amaria, PhD (Hospital for Sick Children, University of Toronto). Health Literacy: Cynthia Latty, RN (Riley Hospital for Children, Indiana University Health); Statistics Consultants: Robert Haley, MD (University of Texas Southwestern), Patrick Monahan, PhD (Richard M. Fairbanks School of Public Health, Indiana University); Participant Recruitment: Robin Bowman, MD (Lurie Children’s Hospital of Chicago, Northwestern University), Timothy J. Brei, MD (Seattle Children’s Hospital, Seattle Children’s Hospital), Jerry Clayton, MD (Children’s Hospital Colorado, University of Colorado), Dominic C. Frimberger, MD (The Children’s Hospital of Oklahoma, University of Oklahoma), Betsy Hopson, MSHA (Children’s Hospital of Alabama, University of Alabama at Birmingham), Jill Mazurek, MD (Indiana University Health), Brandon G. Rocque, MD (Children’s Hospital of Alabama, University of Alabama at Birmingham), Dorota A. Szczepaniak, MD (Riley Hospital for Children, Indiana University Health); Record Entry and Review: Sable Amstutz, MD (Indiana University Health), Kyle Hardacker (Indiana University Health), Zoe King (Indiana University Health), Sophie Mazurek (Indiana University Health), Meredith Metcalf, MD (University of Tennessee Health Sciences Centre), David Yang, BSc (Indiana University Health); Organizations assisting in participant recruitment: International: International Federation for Spina Bifida and Hydrocephalus. United States: Spina Bifida Association, Spina Bifida Associations of Alabama, the Carolinas, Community of Memphis, Connecticut, Georgia, Greater New England, Illinois, Indiana, Kentucky, Nassau County, New Orleans, North Texas, Texas, Washington, Western Pennsylvania, Wisconsin, SB Resource Network, Spina Bifida Connection, National Birth Defects Prevention Network, Disabled Children of Vietnam Veterans. Canada: Spina Bifida & Hydrocephalus Association of Canada, Spina Bifida & Hydrocephalus Association of Ontario, Association de Spina-Bifida et d’Hydrocéphalie du Québec. Europe: Spina Bifida Hydrocephalus Ireland (Ireland), Mid-West Spina Bifida and Hydrocephalus Association (Ireland), Cork Spina Bifida and Hydrocephalus Association (Ireland), Spina bifida Hydrocephalus Information Networking Equality (SHINE, UK), Arbeitsgemeinschaft Spina Bifida und Hydrocephalus e. V. (ASBH, Germany), Swedish National Association for Disabled Children and Young People (FUB) (Sweden), Asociación Bizkaia Elkartea Espina Bífida e Hidrocefalia (Spain), Fundacja Spina (Poland), Spina Bifida ir Hidrocefalija asociacija (SBHA) (Lithuania). Australia and Oceania: Spina Bifida Foundation Victoria (Australia), Spina Bifida Hydrocephalus Queensland (Australia), Spina Bifida Awareness (New Zealand). Middle East: Lebanese Association for Neuromuscular Diseases for Hope (Lebanon). South America: Associação de Espinha Bífida e Hidrocefalia (AEBH, Brazil). Africa: Festus Fajemilo Foundation (Nigeria), National Council for Persons with Physical Disabilities in South Africa (South Africa).
Conflict of interest
Dr. Misseri is a site Investigator and Shelly King is a site Coordinator of the Allergan study 191622-120. None of the other authors declare potential competing personal interests. No authors declare any potential financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Szymanski, K.M., Misseri, R., Whittam, B. et al. QUAlity of Life Assessment in Spina bifida for Adults (QUALAS-A): development and international validation of a novel health-related quality of life instrument. Qual Life Res 24, 2355–2364 (2015). https://doi.org/10.1007/s11136-015-0988-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-015-0988-5