Abstract
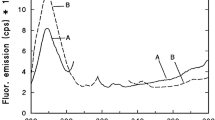
The photoreduction and photooxidation processes of different protochlorophyll(ide) forms were studied in the innermost leaves of cabbage (Brassica oleracea var. capitata L.) under monochromatic irradiations. Room-temperature fluorescence emission spectra were measured from the same leaf spots before and after illumination to follow the wavelength dependence of the photochemical reactions. Short-wavelength light of 7 µmol photons m−2 s−1 (625–630 nm) provoked mainly bleaching, and longer wavelengths (630–640 nm) caused both bleaching and photoreduction, while above 640 nm resulted in basically photoreduction. When bleached leaves were kept in darkness at room temperature, all protochlorophyll(ide) forms regenerated during 72 h. Oxygen-reduced environment decreased the extent of bleaching suggesting the involvement of reactive oxygen species. These results confirm that the short-wavelength, 628 nm absorbing, and 633 nm emitting protochlorophyll(ide) form in etiolated cabbage leaves sensibilizes photooxidation. However, the 628 nm light at low intensities stimulates the photoreduction of the longer wavelength protochlorophyllide forms. Kinetic measurements showed that photoreduction saturates at a low PFD (photon flux density) compared to bleaching, suggesting that the quantum yield of photoreduction is higher than that of bleaching.










Similar content being viewed by others
Abbreviations
- Chl:
-
Chlorophyll
- Chlide:
-
Chlorophyllide
- L-POR:
-
Light-dependent NADPH:protochlorophyllide oxidoreductase enzyme
- Pchl:
-
Protochlorophyll
- Pchlide:
-
Protochlorophyllide
- PFD:
-
Photon flux density
- ROS:
-
Reactive oxygen species
References
Belyaeva OB, Litvin FF (2007) Photoactive pigment-enzyme complexes of chlorophyll precursor in plant leaves. Biochemistry (Moscow) 72:1458–1477
Böddi B, Franck F (1997) Room temperature fluorescence spectra of protochlorophyllide and chlorophyllide forms in etiolated bean leaves. J Photoch Photobio B 41:73–82
Böddi B, Ryberg M, Sundqvist C (1991) The formation of a short-wavelength chlorophyllide form at partial phototransformation of protochlorophyllide in etioplast inner membranes. Photochem Photobiol B 53:667–673
Böddi B, Ryberg M, Sundqvist C (1992) Identification of four universal protochlorophyllide forms in dark-grown leaves by analyses of the 77 K fluorescence emission spectra. J Photoch Photobio 12:389–401
Böddi B, Mc Ewen B, Ryberg M, Sundqvist C (1994) Protochlorophyllide forms in non-greening epicotyls of dark-grown pea (Pisum sativum). Physiol Plant 92:160–170
Böddi B, Kis-Petik K, Kaposi AD, Fidy J, Sundqvist C (1998) The two spectroscopically different short wavelength protochlorophyllide forms in pea epicotyls are both monomeric. Biochim Biophys Acta 1365:531–540
Böddi B, Bóka K, Sundqvist C (2004) Tissue specific protochlorophyll (ide) forms in dark-forced shoots of grapevine (Vitis vinifera L.). Photosynth Res 82:141–150
Busch AW, Montgomery BL (2015) Interdependence of tetrapyrrole metabolism, the generation of oxidative stress and the mitigative oxidative stress response. Redox Biol 4:260–271
Domanskii VP, Rüdiger W (2001) On the nature of the two pathways in chlorophyll formation from protochlorophyllide. Photosynth Res 68:131–139
El Hamouri B, Brouers M, Sironval C (1981) Pathway from photoinactive p 633–628 protochlorophyllide to the p 696–682 chlorophyllide in cucumber etioplast suspension. Plant Sci Lett 21:375–379
Erdei N, Cs Barta, Hideg E, Böddi B (2005) Light-induced wilting and its molecular mechanism in epicotyls of dark-germinated pea (Pisum sativum L.) seedlings. Plant Cell Physiol 46:185–191
Franck F, Schmid GH (1985) The role of NADPH in the reversible phototransformation of chlorophyllide P682 into chlorophyllide P678 in etioplasts of oat. Z Naturforsch C 40:832–838
Franck F, Sperling U, Frick G, Pochert B, van Cleve B, Apel K, Armstrong GA (2000) Regulation of etioplast pigment-protein complexes, inner membrane architecture, and protochlorophyllide a chemical heterogeneity by light-dependent NADPH: protochlorophyllide oxidoreductases A and B. Plant Phys 124(4):1678–1696
Gabruk M, Mysliwa-Kurdziel BJ (2015) Light-dependent protochlorophyllide oxidoreductase: phylogeny, regulation and catalytic properties. Biochemistry-US. doi:10.1021/acs.biochem.5b00704
Hideg É, Vitányi B, Kósa A, Solymosi K, Bóka K, Won S, Inoue Y, Ridge R, Böddi B (2010) Reactive oxygen species from type-I photosensitized reactions contribute to the light-induced wilting of dark-grown pea (Pisum sativum) epicotyls. Physiol Plantarum 138:485–492
Houssier C, Sauer K (1970) Circular dichroism and magnetic circular dichroism of the chlorophyll and protochlorophyll pigments. J Am Chem Soc 92:779–791
Kahn A, Boardman NK, Thorne SW (1970) Energy transfer between protochlorophyllide molecules: evidence for multiple chromophores in the photoactive protochlorophyllide-protein complex in vivo and in vitro. J Mol Biol 48:85–101
Kirsch M, de Groot H (2001) NAD(P)H, a directly operating antioxidant? FASEB J 15:1569–1574
Kósa A, Böddi B (2012) Dominance of a 675 nm chlorophyll(ide) form upon selective 632.8 or 654 nm laser illumination after partial protochlorophyllide phototransformation. Photosynth Res 114:111–120
Kruk J (2005) Occurrence of chlorophyll precursors in leaves of cabbage heads-the case of natural etiolation. J Photoch Photobio B 80:187–194
Lebedev N, Timko M (1999) Protochlorophyllide oxidoreductase B-catalyzed protochlorophyllide photoreduction in vitro: insight into the mechanism of chlorophyll formation in light-adapted plants. P Natl Acad Sci USA 96:9954–9959
Meskauskiene R, Nater M, Goslings D, Kessler F, op den Camp R, Apel K (2001) FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. P Natl Acad Sci USA 98:12826–12831
op den Camp RG, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, Wagner D, Hideg É, Göbel C, Feussner I, Nater M, Apel K (2003) Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15:2320–2332
Richter A, Peter E, Pörs Y, Lorenzen S, Grimm B, Czarnecki O (2010) Rapid dark repression of 5-aminolevulinic acid synthesis in green barley leaves. Plant Cell Physiol 51:670–681
Ryberg M, Sundqvist C (1988) The regular ultrastructure of isolated prolamellar bodies depends on the presence of membrane-bound NADPH-protochlorophyllide oxidoreductase. Physiol Plant 73:18–226
Schoefs B, Garnir HP, Bertrand M (1994) Comparison of the photoreduction of protochlorophyllide to chlorophyllide in leaves and cotyledons from dark-grown bean as a function of age. Photosynth Res 41:405–417
Shibata K (1957) Spectroscopic studies on chlorophyll formation in intact leaves. J Biochem 44:147–173
Solymosi K, Martinez K, Kristóf Z, Sundqvist C, Böddi B (2004) Plastid differentiation and chlorophyll biosynthesis in different leaf layers of white cabbage (Brassica oleracea cv. capitata). Physiol Plant 121:520–529
Szenzenstein A, Kósa A, Böddi B (2008) Biological variability in the ratios of protochlorophyllide forms in leaves and epicotyls of dark-grown pea (Pisum sativum L.) seedlings (A statistical method to resolve complex spectra). J Photoch Photobio B 90:88–94
Tanaka R, Tanaka A (2007) Tetrapyrrole biosynthesis in higher plants. Annu Rev Plant Biol 58:321–346
Vitányi B, Kósa A, Solymosi K, Böddi B (2013) Etioplasts with protochlorophyll and protochlorophyllide forms in the under-soil epicotyl segments of pea (Pisum sativum) seedlings grown under natural light conditions. Physiol Plant 148:307–315
Wilks H, Timko M (1995) A light-dependent complementation system for analysis of NADPH: protochlorophyllide oxidoreductase: identification and mutagenesis of two conserved residues that are essential for enzyme activity. P Natl Acad Sci USA 92(3):724–728
Zhang DW, Yuan S, Xu F, Zhu F, Yuan M, Ye HX, Guo H-Q, Lu X, Yin Y, Lin HH (2014) Light intensity affects chlorophyll synthesis during greening process by metabolite signal from mitochondrial alternative oxidase in Arabidopsis. Plant Cell Environ. doi:10.1111/pce.12438
Acknowledgments
We are grateful to the following former students for their contribution in data collection: Katia Plouznikoff, master student of Université de Bourgogne, Dijon, France; also Valeria Ketykó and Dénes Kleiner undergraduate students of Semmelweis University Budapest, Hungary.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erdei, A.L., Kósa, A., Kovács-Smirová, L. et al. Wavelength-dependent photooxidation and photoreduction of protochlorophyllide and protochlorophyll in the innermost leaves of cabbage (Brassica oleracea var. capitata L.). Photosynth Res 128, 73–83 (2016). https://doi.org/10.1007/s11120-015-0200-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-015-0200-3