Abstract
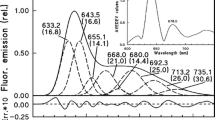
The phototransformation pathways of protochlorophyllide forms were studied in 8–14-day-old leaves of dark-germinated wheat (Triticum aestivum L.) using white, 632.8 nm He–Ne laser and 654 nm laser diode light. The photon flux density (PFD) values (0.75–360 μmol photons m−2 s−1), the illumination periods (20 ms–10 s) and the temperature of the leaves (between −60 °C and room temperature) were varied. The 77 K fluorescence spectra of partially phototransformed leaves showed gradual accumulation or even the dominance of the 675 nm emitting chlorophyllide or chlorophyll form at room temperature with 632.8 nm of PFD less than 200 μmol photons m−2 s−1 or with 654 nm of low PFD (7.5 μmol photons m−2 s−1) up to 1 s. Longer wavelength (685 or 690 nm) emitting chlorophyllide forms appeared at illuminations under −25 °C with both laser lights or at room temperature when the PFD values were higher or the illumination period was longer than above. We concluded that the formation of the 675 nm emitting chlorophyllide form does not indicate the direct photoactivity of the 633 nm emitting protochlorophyllide form; it can derive from 644 and 657 nm forms via instantaneous disaggregation of the newly-produced chlorophyllide complexes. The disaggregation is strongly influenced by the molecular environment and the localization of the complex.





Similar content being viewed by others
Abbreviations
- Chlide:
-
Chlorophyllide
- Cxxx:
-
Chlorophyllide form with fluorescence emission maximum at xxx nm
- Pchlide:
-
Protochlorophyllide
- PFD:
-
Photon flux density
- PLB:
-
Prolamellar body
- POR:
-
NADPH:Pchlide oxidoreductase
- PT:
-
Prothylakoid
- Pxxx:
-
Protochlorophyllide form with fluorescence emission maximum at xxx nm
- ROS:
-
Reactive oxygen species
References
Amirjani MR (2010) Protochlorophyllide spectral forms. Pak J Biol Sci 13:563–576
Amirjani MR, Sundqvist C (2006) Red region excitation spectra of protochlorophyllide in dark-grown leaves from plant species with different proportions of its spectral forms. Photosynthetica 44:83–92
Amirjani M, Sundqvist K, Sundqvist C (2006) Protochlorophyllide and POR development in dark-grown plants with different proportions of short-wavelength and long-wavelength protochlorophyllide spectral forms. Physiol Plant 128:751–762
Avarmaa R, Renge I, Mauring K (1984) Sharp-line structure in the fluorescence and excitation spectra of greening etiolated leaves. FEBS Lett 167:186–190
Belyaeva OB, Litvin FF (1981) Primary reactions of protochlorophyllide into chlorophyllide phototransformation at 77 K. Photosynthetica 15:210–215
Belyaeva OB, Litvin FF (2009) Pathways of formation of pigment forms at the terminal photobiochemical stage of chlorophyll biosynthesis. Biochemistry 74:1535–1544
Belyaeva OB, Litvin FF (2011) Advances in understanding of the primary reactions of protochlorophyll(ide) photoreduction in cells and model systems. J Biophys Chem 2:1–9
Birve SJ, Selstam E, Johansson LB (1996) Secondary structure of NADPH: protochlorophyllide oxidoreductase examined by circular dichroism and prediction methods. Biochem J 317:549–555
Böddi B, Franck F (1997) Room temperature fluorescence spectra of protochlorophyllide and chlorophyllide forms in etiolated bean leaves. J Photochem Photobiol B 41:73–82
Böddi B, Lindsten A, Ryberg M, Sundqvist C (1989) On the aggregational states of protochlorophyllide and its protein complexes in wheat etioplasts. Physiol Plant 76:135–143
Böddi B, Lindsten A, Ryberg M, Sundqvist C (1990) Phototransformation of aggregated forms of protochlorophyllide in isolated etioplast inner membranes. Photochem Photobiol 52:83–87
Böddi B, Ryberg M, Sundqvist C (1991) The formation of a short-wavelength chlorophyllide form at partial phototransformation of protochlorophyllide in etioplast inner membranes. Photochem Photobiol 53:667–673
Böddi B, Ryberg M, Sundqvist C (1992) Identification of four universal protochlorophyllide forms in dark-grown leaves by analyses of the 77 K fluorescence emission spectra. J Photochem Photobiol B 12:389–401
Böddi B, Kis-Petik K, Kaposi AD, Fidy J, Sundqvist C (1998) The two short-wavelength protochlorophyllide forms in pea epicotyls are both monomeric. BBA Bioenerg 1365:531–540
Böddi B, Popovic R, Franck F (2003) Early reactions of light-induced protochlorophyllide and chlorophyllide transformations analyzed in vivo at room temperature with a diode array spectrofluorometer. J Photochem Photobiol B 69:31–39
Böddi B, Loudeche R, Franck F (2005) Delayed chlorophyll accumulation and pigment photodestruction in the epicotyls of dark—grown pea (Pisum sativum). Physiol Plant 125:365–372
Canaani OD, Sauer K (1977) Analysis of the subunit structure of protochlorophyllide holocrome by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Plant Physiol 60:422–429
Cohen CE, Rebeiz CA (1981) Chloroplast biogenesis 34. Spectrofluorometric characterization in situ of the protochlorophyll species in etiolated tissues of higher plants. Plant Physiol 67:98–103
Domanski VP, Rüdiger W (2001) On the nature of the two pathways in chlorophyll formation from protochlorophyllide. Photosynth Res 68:131–139
El Hamouri B, Brouers M, Sironval C (1981) Pathway from photoinactive P633–628 protochlorophyllide to the P696–682 chlorophyllide in cucumber etioplast suspensions. Plant Sci Lett 21:375–379
Eullaffroy P, Popovic R, Franck F (1998) Changes of chlorophyll(ide) fluorescence yield induced by a short light pulse as a probe to monitor the early steps of etioplast phototransformation in dark-grown leaves. Photochem Photobiol 67:676–682
Franck F, Inoue Y (1984) Light-driven reversible transformation of chlorophyllide P696,682 into chlorophyllide P688,678 in illuminated etiolated bean leaves. Photochem Photobiol 8:85–96
Franck F, Bereza B, Böddi B (1999) Protochlorophyllide-NADP+ and protochlorophyllide-NADPH complexes and their regeneration after flash illumination in leaves and etioplast membranes of dark-grown wheat. Photosynth Res 59:53–61
Frank SR (1946) The effectiveness of the spectrum in chlorophyll formation. J Gen Physiol 29:157–179
Griffiths WT (1975) Characterization of the terminal stages of chlorophyll(ide) synthesis in etioplast membrane preparations. Biochem J 152:623–663
Griffiths WT (1978) Reconstitution of chlorophyllide formation by isolated etioplast membranes. Biochem J 174:681–692
Griffiths WT, McHugh T, Blankenship RE (1996) The light intensity dependence of protochlorophyllide photoconversion and its significance to the catalytic mechanism of protochlorophyllide reductase. FEBS Lett 398:235–238
Hendrich W, Bereza B (1993) Spectroscopic characterization of protochlorophyllide and its transformation. Photosynthetica 28:1–16
Heyes DJ, Hunter CN (2005) Making light work of enzyme catalysis: protochlorophyllide oxidoreductase. Trends Biochem Sci 30:642–646
Heyes DJ, Menon BRK, Sakuma M, Scrutton NS (2008) Conformational events during ternary enzyme-substrate complex formation are rate limiting in the catalytic cycle of the light-driven enzyme protochlorophyllide oxidoreductase (POR). Biochemistry 47:10991–10998
Kahn A, Nielsen O (1974) Photoconvertible protochlorophyll(ide)633/650 in vivo: a single species or two species in dynamic equilibrium? Biochim Biophys Acta 333:409–414
Kahn A, Boardman NK, Thorne SW (1970) Energy transfer between protochlorophyllide molecules: evidence for multiple chromophores in the photoactive protochlorophyllide-protein complex in vivo and in vitro. J Mol Biol 48:85–101
Kis-Petik K, Böddi B, Kaposi AD, Fidy J (1999) Protochlorophyllide forms and energy transfer in dark-grown wheat leaves. Studies by conventional and laser excited fluorescence spectroscopy between 10 K and 100 K. Photosynth Res 60:87–98
Klein S, Schiff JA (1972) The correlated appearance of prolamellar bodies, protochlorophyll(ide) species, and the Shibata shift during development of bean etioplasts in the dark. Plant Physiol 49:619–626
Kósa A, Böddi B (2005) Dynamic interconversion and phototransformation processes of protochlorophyllide complexes during greening. Acta Biol Szeged 49:219–220
Kósa A, Márton Zs, Böddi B (2005) Fast phototransformation of the 636 nm emitting protochlorophyllide form in epicotyls of dark-grown pea (Pisum sativum L.). Physiol Plant 124:132–142
Kósa A, Márton Zs, Solymosi K, Bóka K, Böddi B (2006) Aggregation of the 636 nm emitting monomeric protochlorophyllide form into flash-photoactive, oligomeric 644 and 655 nm emitting forms in vitro. BBA Bioenerg 1757:811–820
Koski VM, French CS, Smith JHC (1951) The action spectrum for the transformation of protochlorophyll to chlorophyll a in normal and albino corn seedlings. Arch Biochem Biophys 31:1–17
Krasnovsky AA, Bystrova MI (1980) Self-assembly of chlorophyll aggregated structures. Biosystems 12:181–194
Le Lay P, Böddi B, Kovacevic D, Juneau P, Dewez D, Popovic R (2001) Spectroscopic analysis of desiccation-induced alterations of the chlorophyllide transformation pathway in etiolated barley leaves. Plant Physiol 127:202–211
Lebedev N, Timko MP (1999) Protochlorophyllide oxidoreductase B-catalyzed protochlorophyllide photoreduction in vitro: insight into the mechanism of chlorophyll formation in light-adapted plants. Proc Natl Acad Sci USA 96:9954–9959
Lebedev N, van Cleve B, Armstrong G, Apel K (1995) Chlorophyll synthesis in a deetiolated (det340) mutant of Arabidopsis without NADPH–protochlorophyllide (PChlide) oxidoreductase (POR) A and photoactive PChlide–F655. Plant Cell 7:2081–2090
Lindsten A, Ryberg M, Sundqvist C (1988) The polypeptide composition of highly purified prolamellar bodies and prothylakoids from wheat (Triticum aestivum) as revealed by silver staining. Physiol Plant 72:167–176
Martin GM, Timko MP, Wilks HM (1997) Purification and kinetic analysis of pea (Pisum sativum L.) NADPH: protochlorophyllide oxidoreductase expressed as fusion with maltose-binding protein in Escherichia coli. Biochem J 325:139–145
Mathis P, Sauer K (1972) Circular dichroism studies on the structure and photochemistry of protochlorophyllide and chlorophyllide holochrome. Biochim Biophys Acta 267:498–511
Mathis P, Sauer K (1973) Chlorophyll formation in greening bean leaves during the early stages. Plant Physiol 51:115–119
Mc Ewen B, Seyyedi M, Younis S, Sundqvist C (1996) Formation of short-wavelength chlorophyll(ide) after brief irradiation is correlated with the occurrence of protochlorophyll(ide)636–642 in dark-grown epi- and hypocotyls of bean (Phaseolus vulgaris). Physiol Plant 96:51–58
Michel JM, Sironval C (1977) Shifts to C675–670 and C696–684 in etiolated leaves illuminated with series of brief flashes. Plant Cell Physiol 18:1223–1234
Oliver RP, Griffiths T (1982) Pigment-protein complexes of illuminated etiolated leaves. Plant Physiol 70:1019–1025
Ouazzani Chahdi AM, Schoefs B, Franck F (1998) Isolation and characterization of photoactive complexes of NADPH protochlorophyllide oxidoreductase. Planta 206:673–680
Reinbothe S, Reinbothe C, Apel K, Lebedev N (1996) Evolution of chlorophyll biosynthesis—the challenge to survive photooxidation. Cell 86:703–705
Reinbothe C, El Bakkouri M, Buhr F, Muraki N, Nomata J, Kurisu G, Fujita Y, Reinbothe S (2010) Chlorophyll biosynthesis: spotlight on protochlorophyllide reduction. Trends Plant Sci 15:614–624
Ryberg M, Sundqvist C (1982) Spectral forms of protochlorophyllide in prolamellar bodies and prothylakoids fractioned from wheat etioplasts. Physiol Plant 56:133–138
Ryberg M, Sundqvist C (1988) The regular structure of isolated prolamellar bodies depends ont he presence of membrane-bound NADPH-protochlorophyllide oxidoreductase. Physiol Plant 73:218–226
Schoefs B (2001) The protochlorophyllide-chlorophyllide cycle. Photosynth Res 70:257–271
Schoefs B, Bertrand M, Funk C (2000) Photoactive protochlorophyllide regeneration in cotyledons and leaves from higher plants. Photochem Photobiol 72:660–668
Schoefs B, Franck F (1993) Photoreduction of protochlorophyllide to chlorophyllide in 2-d-old dark-grown bean (Phaseolus vulgaris cv. Commodore) leaves. Comparison with 10-d-old dark-grown (etiolated) leaves. J Exp Bot 44:1053–1057
Schoefs B, Franck F (1998) Chlorophyll synthesis in dark-grown pine primary needles. Plant Physiol 118:1159–1168
Schoefs B, Franck F (2003) Protochlorophyllide reduction: mechanisms and evolution. Photochem Photobiol 78:543–557
Schoefs B, Franck F (2008) The photoenzymatic cycle of NADPH: protochlorophyllide oxidoreductase in primary bean leaves (Phaseolus vulgaris) during the first days of photoperiodic growth. Photosynth Res 96:15–26
Schultz A, Sauer K (1972) Circular dichroism and fluorescence changes accompanying the protochlorophyllide to chlorophyllide transformation in greening leaves and holochrome preparations. BBA Bioenerg 267:320–340
Shalygo NV, Mock HP, Averina NG, Grimm B (1998) Photodynamic action of uroporphyrin and protochlorophyllide in greening barley leaves treated with cesium chloride. J Photochem Photobiol B 42:151–158
Shibata K (1957) Spectroscopic studies on chlorophyll formation in intact leaves. J Biochem 44:147–173
Šiffel P, Lebedev NN, Krasnovskiï AA (1987) Detection of shortwavelength chlorophyll a emission in green leaves. Photosynthetica 21:23–28
Sironval C, Brouers M (1970) The reduction of protochlorophyllide into chlorophyllide. II. The temperature dependence of the P657–647 → P688–676 phototransformation. Photosynthetica 4:38–47
Sironval C, Brouers M (1980) The reduction of protochlorophyllide. VIII. The theory of transfer units. Photosynthetica 14:213–221
Solymosi K, Schoefs B (2008) Prolamellar body: a unique plastid compartment, which does not only occur in dark-grown leaves. In: Schoefs B (ed) Plant cell organelles—selected topics. Research Signpost, Trivandrum, pp 151–202
Sperling U, van Cleve B, Frick G, Apel K, Armstrong GA (1997) Overexpression of light-dependent PORA or PORB in plants depleted of endogenous POR by far-red light enhances seedling survival in white light and protects against photooxidative damage. Plant J 12:649–658
Sperling U, Franck F, van Cleve B, Frick G, Apel K, Armstrong GA (1998) Etioplast differentiation in Arabidopsis: both PORA and PORB restore the prolamellar body and photoactive protochlorophyllide-F655 to the cop1 photomorphogenic mutant. Plant Cell 10:283–296
Sundqvist C, Dahlin C (1997) With chlorophyll from prolamellar bodies to light-harvesting complexes. Physiol Plant 100:748–759
Virgin HI (1986) Action spectra for chlorophyll formation during greening of wheat leaves in continuous light. Physiol Plant 66:277–282
Virgin HI (1993) Effectiveness of light of different wavelengths to induce chlorophyll biosynthesis in rapidly and slowly greening tissues. Physiol Plant 89:761–766
Wiktorsson B, Engdahl S, Zhong LB, Böddi B, Ryberg M, Sundqvist C (1993) The effect of cross-linking of the subunits of NADPH-protochlorophyllide oxidoreductase on the aggregational state of protochlorophyllide. Photosynthetica 29:205–218
Acknowledgments
We are grateful to late Prof. Christer Sundqvist for his valuable suggestions and for providing us with isolated etioplast inner membrane preparations. A. Kósa is grateful for the Ferenc Deák Scholarship of the Hungarian Ministry of Education and Culture (DFÖ 0021/2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kósa, A., Böddi, B. Dominance of a 675 nm chlorophyll(ide) form upon selective 632.8 or 654 nm laser illumination after partial protochlorophyllide phototransformation. Photosynth Res 114, 111–120 (2012). https://doi.org/10.1007/s11120-012-9782-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-012-9782-1