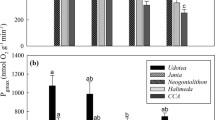
Abstract
Productivity of most macroalgae is not currently considered limited by dissolved inorganic carbon (DIC), as the majority of species have CO2-concentrating mechanisms (CCM) allowing the active uptake of DIC. The alternative, diffusive uptake of CO2 (non-CCM), is considered rare (0–9 % of all macroalgal cover in a given ecosystem), and identifying species without CCMs is important in understanding factors controlling inorganic carbon use by eukaryotic algae. CCM activity has higher energetic requirements than diffusive CO2 uptake, therefore when light is low, CCM activity is reduced in favour of diffusive CO2 uptake. We hypothesized that the proportional cover of macroalgae without CCMs (red and green macroalgae) would be low (<10 %) across four sites in Tasmania, southern Australia at two depths (4–5 and 12–14 m); the proportion of species lacking CCMs would increase with decreasing depth; the δ13C values of macroalgae with CCMs would be more depleted with depth. We found the proportion of non-CCM species ranged from 0 to 90 % and included species from all three macroalgal phyla: 81 % of red (59 species), 14 % of brown (three species) and 29 % of green macroalgae (two species). The proportion of non-CCM species increased with depth at three of four sites. 35 % of species tested had significantly depleted δ13C values at deeper depths. Non-CCM macroalgae are more abundant in some temperate reefs than previously thought. If ocean acidification benefits non-CCM species, the ramifications for subtidal macroalgal assemblages could be larger than previously considered.




Similar content being viewed by others
References
Axelsson L (1988) Changes in pH as a measure of photosynthesis by marine macroalgae. Mar Biol 97:287–294
Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH. Nature 425:365
Ciais P, Sabine CL, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J, Heimann M, Jones C, Le Quêrê RB, Myneni RB, Piao S, Thornton P (2013) Carbon and other biogeochemical cycles. In: Stocker TF, Qin D, Plattner G-K et al (eds) Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Clementson LA, Parslow JS, Turnbull AR, Bonham PI (2004) Properties of light absorption in a highly coloured estuarine system in south-east Australia which is prone to blooms of the toxic dinoflagellate Gymondinium catenatum. Estuar Coast Shelf Sci 60:101–112
Connell SD, Kroeker KJ, Fabricius KE, Kline DI, Russell BD (2013) The other ocean acidification problem: CO2 as a resource amongst competitors for ecosystem dominance. Phil Trans R Soc B 368:20120442. http://dx.doi.org/20120410.20121098/rstb.20122012.20120442
Cornelisen CD, Wing SR, Clark KL, Bowman MH, Frew RD, Hurd CL (2007) Patterns in the δ13C and δ15N signature of Ulva pertusa: interaction between physical gradients and nutrient source pools. Limnol Oceanogr 52:820–832
Cornwall CE, Hepburn CD, Pritchard DW, McGraw CM, Currie KI, Hunter KA, Hurd CL (2012) Carbon-use strategies in macroalgae: differential responses to lowered pH and implications for ocean acidification. J Phycol 48:137–144
Dickson AG, Sabine CL, Christian JR (2007) Guide to best practices for Ocean CO2 measurements. North Pacific Marine Science Organization, Sidney
Dunton KH (2001) δ15N and δ13C measurements of Antarctic Peninsula fauna: trophic relationships and assimilation of benthic seaweeds. Am Zool 41(1):99–112
Fischer G, Wiencke C (1992) Stable carbon isotope composition, depth distribution and fate of macroalgae from the Antarctic Peninsula region. Polar Biol 12:341–348
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56:99–131
Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM, Rowley SJ, Tedesco D, Buia MC (2008) Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454:96–99
Hepburn CD, Pritchard DW, Cornwall CE, McLeod RJ, Beardall J, Raven JA, Hurd CL (2011) Diversity of carbon use strategies in a kelp forest community: implications for a high CO2 ocean. Glob Change Biol 17:2488–2497
Kevekordes K, Holland D, Häbner N, Jenkins S, Kos R, Roberts S, Raven JA, Scrimgeour CM, Shelly K, Stojkovic S, Beardall J (2006) Inorganic carbon acquisition by eight species of Caulerpa (Caulerpaceae, Chlorophyta). Phycologia 45:442–449
Korb RE, Raven JA, Johnston AM, Leftley JW (1996) Effects of cell size and specific growth rate on stable carbon isotope discrimination by two species of marine diatom. Mar Ecol Prog Ser 143:283–288
Kroeker KJ, Kordas RL, Crim RN, Hendriks IE, Ramajo L, Singh GG, Duarte CM, Gattuso JP (2013) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Change Biol 19(6):1884–1896
Kübler JE, Raven JA (1994) Consequences of light limitation for carbon acquisition in three rhodophytes. Mar Ecol Prog Ser 110:203–209
Kübler JE, Johnston AM, Raven JA (1999) The effects of reduced and elevated CO2 and O2 on the seaweed Lomentaria articulata. Plant, Cell Environ 22:1303–1310
Lee D, Carpenter SJ (2001) Isotopic disequilibrium in marine calcareous algae. Chem Geol 172:307–329
Lepoint G, Nyssen F, Gobert S, Dauby P, Bouquegneau JM (2000) Relative impact of a seagrass bed and its adjacent epilithic algal community in consumer diets. Mar Biol 136:513–518
Lüning K, Dring MJ (1979) Continuous underwater light measurements near Helgoland (North Sea) and its significance for characteristic light limits in the sublittoral region. Helgol Mar Res 32:403–424
Maberly SC (1990) Exogenous sources of inorganic carbon for photosynthesis by marine macroalgae. Phycologia 26:439–449
Maberly SC, Madsen TV (1998) Affinity for CO2 in relation to the ability go freshwater macrophytes to use HCO3 −. Funct Ecol 12:99–106
Maberly SC, Berthelot SA, Stott AW, Gontero B (2014) Adaptation by macrophytes to inorganic carbon down river with naturally variable concentrations of CO2. J Plant Phys 172:120–127
Marconi M, Giordano M, Raven JA (2011) Impact of taxonomy, geography, and depth on δ13C and δ15N variation in a large collection of macroalgae. J Phycol 47:1023–1035
Murru M, Sandgren CD (2004) Habitat matters for inorganic carbon acquisition in 38 species of red macroalgae (Rhodophyta) from Puget Sound, Washington, USA. J Phycol 40:837–845
Nelson W, Neill K, D’Archino R, Anderson T, Beaumont J, Dalen J (2014) Beyond diving depths: deepwater macroalgae in the New Zealand region. Mar Biodiv. doi:10.1007/s1256-014-0293-5
R Core Development Team (2008) R: a language and environment for statistical computing. 2.7.0 edn. R Foundation for Statistical Computing, Vienna
Raven JA (1991) Physiology of inorganic C acquisition and implications for resource use efficiency by marine phytoplankton: relation to increased CO2 and temperature. Plant, Cell Environ 14:779–794
Raven JA (1997) Putting the C in phycology. Eur J Phycol 32:319–333
Raven JA (2010) Inorganic carbon acquisition by eukaryotic algae: four current questions. Photosynth Res 106:123–134
Raven JA, Beardall J (2014) CO2 concentrating mechanisms and environmental change. Aquat Bot 118:24–37
Raven JA, Johnston AM, Kübler JE, Korb R, McInroy SG, Handley LL, Scrimgeour CM, Walker DI, Beardall J, Clayton MN, Vanderklift MA, Fredriksen S, Dunton KH (2002a) Seaweeds in cold seas: evolution and carbon acquisition. Ann Bot 90:525–536
Raven JA, Johnston AM, Kübler JE, Korb RE, McInroy SG, Handley LL, Scrimgeour CM, Walker DI, Beardall J, Vanderklift MA, Fredriksen S, Dunton KH (2002b) Mechanistic interpretation of carbon isotope discrimination by marine macroalgae and seagrasses. Funct Plant Biol 29:355–378
Raven JA, Ball LA, Beardall J, Giordano M, Maberly SC (2005a) Algae lacking carbon-concentrating mechanisms. Can J Bot 83:879–890
Raven JA, Caldeira K, Elderfield H, Hoegh-Guldberg O, Liss P, Riebesell U, Shepherd J, Turley C, Watson A (2005b) Ocean acidification due to increasing atmospheric carbon dioxide. The Royal Society, London
Raven JA, Giodarno M, Beardall J, Maberly SC (2011) Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynth Res 109:281–296
Raven JA, Beardall J, Giordano M (2014) Energy costs of carbon dioxide concentrating mechanisms in aquatic organisms. Photosynth Res 121:111–124
Runcie JW, Gurgel CFD, McDermid KJ (2008) In situ photosynthetic rates of tropical marine macroalgae at their lower depth limit. Eur J Phycol 43(4):377–388
Scott FJ (2012) Rare marine macroalgae of southern Australia. University of Tasmania, Hobart
Silberfeld T, Leight JW, Verbruggen H, Cruaud C, de Reviers B, Rousseau F (2010) A multi-locus time-calibrated phylogeny of the brown algae (Heterokonta, Ochrophyta, Phaeophyceae): investigating the evolutionary nature of the “brown algal crown radiation”. Mol Phylogenet Evol 56:659–674
Steneck RS, Graham MH, Bourque BJ, Corbett P, Erlandson JM, Estes JA, Tegner MJ (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ Conserv 29:436–459
Tegner MJ, Dayton PK (2000) Ecosystem effects of fishing in kelp forest communities. ICES J Mar Sci 57:579–589
Wiencke C, Fischer G (1990) Growth and stable carbon isotop composition of cold-water macroalgae in relation to light and temperature. Mar Ecol Prog Ser 65:283–292
Acknowledgments
We thank S. Ling, A. Fowles, F. Scott, D. Britton, E. Flukes, C. Layton, M. Cameron, M. Taksumi, J. Kean and T. Baulch for assistance in the field, laboratory or with identification of species. We also thank E. Flukes and C. Johnson for providing the irradiance estimates for Fortescue Bay. Samples were collected using the Department of Primary Industries, Parks, Water & Environment Tasmania permit number 13120.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cornwall, C.E., Revill, A.T. & Hurd, C.L. High prevalence of diffusive uptake of CO2 by macroalgae in a temperate subtidal ecosystem. Photosynth Res 124, 181–190 (2015). https://doi.org/10.1007/s11120-015-0114-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-015-0114-0