Abstract
Aims
Woody encroachment and subsequent brush management aimed at reducing woody plant cover can alter soil organic carbon (SOC) pools. However, brush management influences on the sources and stability of SOC is unknown. Using a space-for-time approach in a site with closely co-located patches representing unencroached grassland, woody encroachment, and brush management, we coupled stable isotopes and plant-derived biomarkers to quantify how woody encroachment alters input sources and stability of SOC and how these patterns change with brush management.
Methods
Stable isotopes, lignin-derived phenols, substituted fatty acids, and carbon content of density fractions were measured in soils collected in shrub canopy interspaces, under live shrubs, and under shrubs killed 8 or 52 y previously.
Results
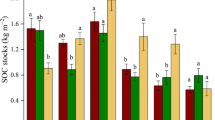
Bulk SOC and C3-derived SOC were higher in shallow soil (0–5 cm) under live shrubs than in interspace soil. Long-term brush management showed a decline in total SOC stocks, substituted fatty acids, and C3-derived SOC that were associated with the soil light fraction. Despite declines in bulk SOC following brush management, accumulated C3-derived SOC pools in the dense fraction remained largely stable following brush management.
Conclusions
Woody encroachment increases the proportion of poorly-protected light fraction and loosely-mineral associated SOC derived from leaf litter, which is lost within several decades after brush management. However, woody encroachment produced a stabilized mineral-bound pool of C3- and C4-derived SOC that remained 52 years after brush management, suggesting that woody encroachment has the potential for long-term SOC stabilization.






Similar content being viewed by others
References
Alvarez JA, Villagra PE, Rossi BE, Cesca EM (2009) Spatial and temporal litterfall heterogeneity generated by woody species in the Central Monte desert. Plant Ecol 205:295–303. doi:10.1007/s11258-009-9618-z
Ansley RJ, Kramp BA, Jones DL (2003) Converting mesquite thickets to savanna through foliage modification with clopyralid. J Range Manag 56:72–80. http://uvalde.tamu.edu/jrm/jrmhome.htm
Archer SR, Davies KW, Fulbright TE (2011) Brush Management as a Rangeland Conservation Strategy: A Critical Evaluation. Conservation Benefits of Rangeland Practices pp:105–170
Asner GP, Elmore AJ, Olander LP, et al. (2004) Grazing systems, ecosystem responses, and global change. Annu Rev Environ Resour 29:261–299. doi:10.1146/annurev.energy.29.062403.102142
Bai E, Boutton TW, Liu F, et al. (2012) Spatial variation of soil delta 13C and its relation to carbon input and soil texture in a subtropical lowland woodland. Soil Biol Biochem 44:102–112. doi:10.1016/j.soilbio.2011.09.013
Barger NN, Archer SR, Campbell JL, et al (2011) Woody plant proliferation in North American drylands: A synthesis of impacts on ecosystem carbon balance. J Geophys Res 116:G00 K07. doi:10.1029/2010JG001506
Bechtold HA, Inouye RS (2007) Distribution of carbon and nitrogen in sagebrush steppe after six years of nitrogen addition and shrub removal. J Arid Environ 71:122–132. doi:10.1016/j.jaridenv.2007.02.004
Breckenfield DJ, Robinett D (1997) Soil and range resource inventory of the Santa Rita Experimental Range, Pima County, Arizona, special report
Browning DM, Archer SR (2011) Protection from livestock fails to deter shrub proliferation in a desert landscape with a history of heavy grazing. Ecol Appl 21:1629–1642. doi:10.1890/10-0542.1
Cable DR (1971) Lehmann lovegrass on the Santa Rita Experimental Range, 1937-1968. J Range Manag 24:17–21
Carrington EM, Hernes PJ, Dyda RY, et al. (2012) Biochemical changes across a carbon saturation gradient: Lignin, cutin, and suberin decomposition and stabilization in fractionated carbon pools. Soil Biol Biochem 47:179–190. doi:10.1016/j.soilbio.2011.12.024
Collins CDH, Kautz MA, Tiller R, et al. (2015) Development of an integrated multiplatform approach for assessing brush management conservation efforts in semiarid rangelands. J Appl Remote Sens 9:1–15
Connin SL, Virginia RA, Chamberlain CP (1997) Carbon isotopes reveal soil organic matter dynamics following arid land shrub expansion. Oecologia 110:374–386
Cotrufo MF, Wallenstein MD, Boot CM, et al. (2013) The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob Chang Biol 19:988–995. doi:10.1111/gcb.12113
Creamer CA, Filley TR, Boutton TW, et al. (2011) Controls on soil carbon accumulation during woody plant encroachment: Evidence from physical fractionation, soil respiration, and d13C of respired CO2. Soil Biol Biochem 43:1678–1687. doi:10.1016/j.soilbio.2011.04.013
Creamer CA, Filley TR, Boutton TW (2013) Long-term incubations of size and density separated soil fractions to inform soil organic carbon decay dynamics. Soil Biol Biochem 57:496–503. doi:10.1016/j.soilbio.2012.09.007
Crow SE, Swanston CW, Lajtha K, et al. (2007) Density fractionation of forest soils: methodological questions and interpretation of incubation results and turnover time in an ecosystem context. Biogeochemistry 85:69–90. doi:10.1007/s10533-007-9100-8
Crow SE, Filley TE, McCormick M, Szlavecz K, Stott DE, Gamblin D, and Conyers G (2009) Earthworms, standage, and species composition interact to influence particulate organic matter chemistry during forest succession. Biogeochemistry 92:61–82
Daryanto S, Eldridge DJ, Throop HL (2013) Managing semi-arid woodlands for carbon storage: Grazing and shrub effects on above- and belowground carbon. Agric Ecosyst Environ 169:1–11. doi:10.1016/j.agee.2013.02.001
DeGraaff M-A, Throop HL, Verburg PS, et al. (2014) A synthesis of climate and vegetation cover effects on biogeochemical cycling in shrub-dominated drylands. Ecosystems 17:931–945
DeMarco J, Mack MC, Bret-Harte MS (2014) Effects of arctic shrub expansion on biophysical vs. biogeochemical drivers of litter decomposition. Ecology 95:1861–1875. doi:10.1890/13-2221.1
Dungait JAJ, Hopkins DW, Gregory AS, Whitmore AP (2012) Soil organic matter turnover is governed by accessibility not recalcitrance. Glob Chang Biol 18:1781–1796. doi:10.1111/j.1365-2486.2012.02665.x
Dzurec RS, Boutton TW, Caldwell MM, Smith BN (1985) Carbon isotope ratios of soil organic matter and their use in assessing community composition changes in Curlew Valley, Utah. Oecologia 56:17–24
Ehleringer JR, Buchmann N, Flanagan LB (2000) Carbon isotope ratios in belowground carbon cycle processes. Ecol Appl 10:412–422
Eldridge DJ, Bowker MA, Maestre FT, et al. (2011) Impacts of shrub encroachment on ecosystem structure and functioning: towards a global synthesis. Ecol Lett 14:709–722. doi:10.1111/j.1461-0248.2011.01630.x
Field JP, Breshears DD, Whicker JJ, Zou CB (2012) Sediment capture by vegetation patches: Implications for desertification and increased resource redistribution. J Geophys Res 117:G01033. doi:10.1029/2011JG001663
Filley TR, Boutton TW, Liao JD, et al. (2008) Chemical changes to nonaggregated particulate soil organic matter following grassland-to-woodland transition in a subtropical savanna. J Geophys Res 113:G03009. doi:10.1029/2007JG000564
Goni MA, Hedges JI (1990a) Potential applications of cutin-derived CuO reaction products for discriminating vascular plant sources in natural environments. Geochim Cosmochim Acta 54:3073–3081
Goni MA, Hedges JI (1990b) Cutin-derived CuO reaction products from purified cuticles and tree leaves. Geochim Cosmochim Acta 54:3065–3072
Harris D, Horwáth WR, van Kessel C (2001) Acid fumigation of soils to remove carbonates prior to total organic carbon or CARBON-13 isotopic analysis. Soil Sci Soc Am J 65:1853. doi:10.2136/sssaj2001.1853
Hedges JI, Ertel JR (1982) Characterization of lignin by gas capillary chromatography of cupric oxide oxidation products. Anal Chem 54:174–178
Hewins DB, Archer SR, Okin GS, et al. (2013) Soil-litter mixing accelerates decomposition in a Chihuahuan Desert grassland. Ecosystems 16:183–195
Huxman TE, Snyder KA, Tissue D, et al. (2004) Precipitation pulses and carbon fluxes in semiarid and arid ecosystems. Oecologia 141:254–268. doi:10.1007/s00442-004-1682-4
Kemp PR, Reynolds JF, Virginia RA, Whitford WG (2003) Decomposition of leaf and root litter of Chihuahuan desert shrubs: effects of three years of summer drought. J Arid Environ 53:21–39. doi:10.1006/jare.2002.1025
Krull ES, Bestland EA, Gates WP (2002) Soil organic matter decomposition and turnover in a tropical Ultisol: evidence from delta 13C, delta 15N and geochemistry. Radiocarbon 44:93–112
Liao JD, Boutton TW (2008) Soil microbial biomass response to woody plant invasion of grassland. Soil Biol Biochem 40:1207–1216. doi:10.1016/j.soilbio.2007.12.018
Liao JD, Boutton TW, Jastrow JD (2006a) Organic matter turnover in soil physical fractions following woody plant invasion of grassland: Evidence from natural 13C and 15N. Soil Biol Biochem 38:3197–3210. doi:10.1016/j.soilbio.2006.04.004
Liao JD, Boutton TW, Jastrow JD (2006b) Storage and dynamics of carbon and nitrogen in soil physical fractions following woody plant invasion of grassland. Soil Biol Biochem 38:3184–3196. doi:10.1016/j.soilbio.2006.04.003
Martin BSC, Ward DE (1966) Using aerial applications-two annual sprays control mesquite. Progress Agric Ariz 18:20–21
McClaran MP, Ffolliotte PF, Edminster CB (2003) Santa Rita Experimental Range: 100 Years (1903 to 2003) of Accomplishments and Contributions. Conference Proceedings October 30-November 1, 2003 Tucson, AZ. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Ogden, Utah, pp 1–197
McClaran MP, McMurtry CR, Archer SR (2013) A tool for estimating impacts of woody encroachment in arid grasslands: Allometric equations for biomass, carbon and nitrogen content in Prosopis velutina. J Arid Environ 88:39–42. doi:10.1016/j.jaridenv.2012.08.015
McClaran MP, Moore-Kucera J, Martens DA, et al. (2008) Soil carbon and nitrogen in relation to shrub size and death in a semi-arid grassland. Geoderma 145:60–68. doi:10.1016/j.geoderma.2008.02.006
Miwa CT, Reuter RJ (2010) Persistence of Western Juniper (Juniperus occidentalis) resource islands following canopy removal. Northwest Sci 84:361–368
Mun HT, Whitford WG (1998) Changes in mass and chemistry of plant roots during long-term decomposition on a Chihuahuan Desert watershed. Biol Fertil Soils 26:16–22
Neff JC, Barger NN, Baisden WT, et al. (2009) Soil carbon storage responses to expanding pinyon-juniper populations in southern Utah. Ecol Appl 19:1405–1416
Opsahl S, Benner R (1995) Early diagenesis of vascular plant tissues: Lignin and cutin decomposition and biogeochemical implications. Geochim Cosmochim Acta 59:4889–4904. doi:10.1016/0016-7037(95)00348-7
Otto A, Simpson MJ (2006) Evaluation of CuO oxidation parameters for determining the source and stage of lignin degradation in soil. Biogeochemistry 80:121–142. doi:10.1007/s10533-006-9014-x
Pacala SW, Hurtt GC, Baker D, et al. (2001) Consistent land- and atmosphere-based U.S. carbon sink estimates. Science 292:2316–2320. doi:10.1126/science.1057320
Phillips DL, Gregg JW (2001) Uncertainty in source partitioning using stable isotopes. Oecologia 127:171–179. doi:10.1007/s004420000578
Phillips DL, Johnson MG, Tingey DT, et al. (2006) Effects of elevated CO2 on fine root dynamics in a Mojave Desert community: A FACE study. Glob Chang Biol 12:61–73. doi:10.1111/j.1365-2486.2005.01085.x
Riederer M, Matske K, Ziegler F, Kogel-Knabner I (1993) Occurrence, distribution and fate of the lipid plant biopolymers cutin and suberin in temperature forest soils. Org Geochem 20:1063–1076
Schlesinger WH, Reynolds JF, Cunningham GL, et al (1990) Biological feedbacks in global desertification. Science (80-) 247:1043–1048.
Schmidt MWI, Torn MS, Abiven S, et al. (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56. doi:10.1038/nature10386
Schussman H, Geiger E, Mau-Crimmins T, Ward J (2006) Spread and current potential distribution of an alien grass, Eragrostis lehmanniana Nees, in the southwestern USA: comparing historical data and ecological niche models. Divers Distrib 12:582–592. doi:10.1111/j.1366-9516.2006.00268.x
Smith JG, Eldridge DJ, Throop HL (2012) Landform and vegetation patch type moderate the effects of grazing-induced disturbance on carbon and nitrogen pools in a semi-arid woodland. Plant Soil 360:405–419. doi:10.1007/s11104-012-1288-2
Sollins P, Kramer MG, Swanston C, et al. (2009) Sequential density fractionation across soils of contrasting mineralogy: evidence for both microbial- and mineral-controlled soil organic matter stabilization. Biogeochemistry 96:209–231. doi:10.1007/s10533-009-9359-z
Sollins P, Swanston C, Kleber M, et al. (2006) Organic C and N stabilization in a forest soil: Evidence from sequential density fractionation. Soil Biol Biochem 38:3313–3324. doi:10.1016/j.soilbio.2006.04.014
Song X, Peng C, Jiang H, et al. (2013) Direct and Indirect Effects of UV-B Exposure on Litter Decomposition: A Meta-Analysis. PLoS One 8:e68858. doi:10.1371/journal.pone.0068858
Stevenson BA, Kelly EF, McDonald EV, Busacca AJ (2005) The stable carbon isotope composition of soil organic carbon and pedogenic carbonates along a bioclimatic gradient in the Palouse region, Washington State, USA. Geoderma 124:37–47. doi:10.1016/j.geoderma.2004.03.006
Thompson TL, Zaady E, Huancheng P, et al. (2006) Soil C and N pools in patchy shrublands of the Negev and Chihuahuan Deserts. Soil Biol Biochem 38:1943–1955. doi:10.1016/j.soilbio.2006.01.002
Throop HL, Archer SR (2007) Interrelationships among shrub encroachment, land management, and litter decomposition in a semidesert grassland. Ecol Appl 17:1809–1823
Throop HL, Archer SR (2008) Shrub (Prosopis velutina) encroachment in a semidesert grassland: spatial-temporal changes in soil organic carbon and nitrogen pools. Glob Chang Biol 14:2420–2431. doi:10.1111/j.1365-2486.2008.01650.x
Throop HL, Archer SR, HC Monger, S Waltman. 2012. When bulk density matters: Implications for estimating soil organic pools in rocky soils. Journal of Arid Environments 77:66–71
Throop HL, Lajtha K, Kramer M (2013) Density fractionation and 13C reveal changes in soil carbon following woody encroachment in a desert ecosystem. Biogeochemistry 112:409–422
Tiedemann AR, Klemmedson JO (1986) Long-term effects of mesquite removal on soil characteristics: I. Nutrients and bulk density. Soil Sci Soc Am J 50:472–475
Tiedemann AR, Klemmedson JO (1977) Effect of mesquite trees on vegetation and soils in the desert grassland. J Range Manag 30:361–367
Tiedemann AR, Klemmedson JO (2004) Responses of desert grassland vegetation to mesquite removal and regrowth. J or Range Manag 57:455–465
Werth M, Kuzyakov Y (2010) 13C fractionation at the root–microorganisms–soil interface: A review and outlook for partitioning studies. Soil Biol Biochem 42:1372–1384. doi:10.1016/j.soilbio.2010.04.009
Whitford WG, Kay FR (1999) Biopedturbation by mammals in deserts: a review. J Arid Environ 41:203–230
Whitford WG, Stinnett K, Anderson J (1988) Decomposition of roots in a Chihuahuan Desert ecosystem. Oecologia 75:8–11
Yavitt JB, Smith EL (1983) Spatial patterns of mesquite and associated herbaceous species in an Arizona Desert Grassland. Am J Bot 109:89–93
Zhang T-H (2006) A leguminous shrub (Caragana microphylla) in semiarid sandy soils of North China. Pedosphere 16:319–325
Acknowledgments
This research was supported by US National Science Foundation grant DEB 0953864. Thanks to J.G. Smith for help with soil collection and M. R. Bravo-Garza, K. Slown, and C. Elam for help with sample processing. D. Gamblin and N. Louden helped with the CuO oxidation analyses. We appreciate constructive comments on a previous version of the manuscript from J. Schafer and two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ingrid Koegel-Knabner.
Electronic supplementary material
ESM 1
(DOCX 9624 kb)
Rights and permissions
About this article
Cite this article
DeMarco, J., Filley, T. & Throop, H.L. Patterns of woody plant-derived soil carbon losses and persistence after brush management in a semi-arid grassland. Plant Soil 406, 277–293 (2016). https://doi.org/10.1007/s11104-016-2880-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2880-7