Abstract
Background and aims
Climate change alters regional plant species distributions, creating new combinations of litter species and soil communities. Biogeographic patterns in microbial communities relate to dissimilarity in microbial community function, meaning novel litters to communities may decompose differently than predicted from their chemical composition. Therefore, the effect of a litter species in the biogeochemical cycle of its current environment may not predict patterns after migration. Under a tree migration sequence we test whether litter quality alone drives litter decomposition, or whether soil communities modify quality effects.
Methods
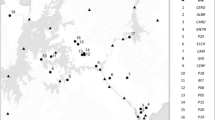
Litter and soils were sampled across an elevation gradient of different overstory species where lower elevation species are predicted to migrate upslope. We use a common garden, laboratory microcosm design (soil community x litter environment) with single and mixed-species litters.
Results
We find significant litter quality and microbial community effects (P < 0.001), explaining 47 % of the variation in decomposition for mixed-litters.
Conclusion
Soil community effects are driven by the functional breadth, or historical exposure, of the microbial communities, resulting in lower decomposition of litters inoculated with upslope communities. The litter x soil community interaction suggests that litter decomposition rates in forests of changing tree species composition will be a product of both litter quality and the recipient soil community.


Similar content being viewed by others
References
Adair EC, Parton WJ, Del Grosso SJ, Silver WL, Harmon ME, Hall SA, Burke IC, Hart SC (2008) Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Glob Change Biol 14(11):2636–2660. doi:10.1111/j.1365-2486.2008.01674.x
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79(3):439–449
Ayres E, Steltzer H, Berg B, Wall DH (2009) Soil biota accelerate decomposition in high-elevation forests by specializing in the breakdown of litter produced by the plant species above them. J Ecol 97(5):901–912. doi:10.1111/j.1365-2745.2009.01539.x
Bardgett RD, Shine A (1999) Linkages between plant litter diversity, soil microbial biomass and ecosystem function in temperate grasslands. Soil Biol Biochem 31(2):317–321. doi:10.1016/s0038-0717(98)00121-7
Butenschoen O, Scheu S, Eisenhauer N (2011) Interactive effects of warming, soil humidity and plant diversity on litter decomposition and microbial activity. Soil Biol Biochem 43(9):1902–1907. doi:10.1016/j.soilbio.2011.05.011
Carpenter SR (1996) Microcosm experiments have limited relevance for community and ecosystem ecology. Ecology 77(3):677–680
Chapin FS, Torn MS, Tateno M (1996) Principles of ecosystem sustainability. Am Nat 148(6):1016–1037
Chapman SK, Newman GS (2010) Biodiversity at the plant-soil interface: microbial abundance and community structure respond to litter mixing. Oecologia 162(3):763–769. doi:10.1007/s00442-009-1498-3
Cornelissen JHC, Lavorel S, Garnier E, Diaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51(4):335–380. doi:10.1071/bt02124
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Perez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Diaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11(10):1065–1071. doi:10.1111/j.1461-0248.2008.01219.x
Cusack DF, Chou WW, Yang WH, Harmon ME, Silver WL, Lidet T (2009) Controls on long-term root and leaf litter decomposition in neotropical forests. Glob Change Biol 15(5):1339–1355. doi:10.1111/j.1365-2486.2008.01781.x
de Wit R, Bouvier T (2006) ‘Everything is everywhere, but, the environment selects’; what did Baas Becking and Beijerinck really say? Environ Microbiol 8(4):755–758. doi:10.1111/j.1462-2920.2006.01017.x
Frey SD, Lee J, Melillo JM, Six J (2012) The temperature response of soil microbial efficiency and its feedback to climate. Nat Clim Chang. doi:10.1038/NCLIMATE1796
Gartner TB, Cardon ZG (2004) Decomposition dynamics in mixed-species leaf litter. Oikos 104(2):230–246. doi:10.1111/j.0030-1299.2004.12738.x
Gessner MO, Swan CM, Dang CK, McKie BG, Bardgett RD, Wall DH, Hattenschwiler S (2010) Diversity meets decomposition. Trends Ecol Evol 25(6):372–380
Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ (2000) Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Glob Change Biol 6(7):751–765
Hanson CA, Fuhrman JA, Horner-Devine MC, Martiny JBH (2012) Beyond biogeographic patterns: processes shaping the microbial landscape. Nat Rev Microbiol. doi:10.1038/nrmicro2795
Hoorens B, Aerts R, Stroetenga M (2003) Does initial litter chemistry explain litter mixture effects on decomposition? Oecologia 137(4):578–586. doi:10.1007/s00442-003-1365-6
Hoorens B, Coomes D, Aerts R (2010) Neighbour identity hardly affects litter-mixture effects on decomposition rates of New Zealand forest species. Oecologia 162(2):479–489. doi:10.1007/s00442-009-1454-2
IPCC (2007) Climate change 2007: synthesis report. Contribution of working groups I, II, and III to the fourth assessment report of the intergovernmental panel on climate change. IPCC, Geneva
Iverson LR, Prasad AM (2001) Potential changes in tree species richness and forest community types following climate change. Ecosystems 4(3):186–199
Jacob M, Weland N, Platner C, Schaefer M, Leuschner C, Thomas FM (2009) Nutrient release from decomposing leaf litter of temperate deciduous forest trees along a gradient of increasing tree species diversity. Soil Biol Biochem 41(10):2122–2130. doi:10.1016/j.soilbio.2009.07.024
Jacob M, Viedenz K, Polle A, Thomas FM (2010) Leaf litter decomposition in temperate deciduous forest stands with a decreasing fraction of beech (Fagus sylvatica). Oecologia 164(4):1083–1094. doi:10.1007/s00442-010-1699-9
Kamei J, Barik SK, Pandey HN (2009) Interspecific variation in leaf litter production, decomposition, and nitrogen and phosphorus loss from decomposing leaves in a humid subtropical forest ecosystem of northeastern India. Can J For Res-Rev Can Rech For 39(10):1797–1805. doi:10.1139/x09-104
Kang H, Kang S, Lee D (2009) Variations of soil enzyme activities in a temperate forest soil. Ecol Res 24(5):1137–1143. doi:10.1007/s11284-009-0594-5
Keiser AD, Strickland MS, Fierer N, Bradford MA (2011) The effect of resource history on the functioning of soil microbial communities is maintained across time. Biogeosciences 8(6):1477–1486. doi:10.5194/bg-8-1477-2011
Laganiere J, Pare D, Bradley RL (2010) How does a tree species influence litter decomposition? Separating the relative contribution of litter quality, litter mixing, and forest floor conditions. Can J For Res-Rev Can Rech For 40(3):465–475. doi:10.1139/x09-208
Langenheder S, Prosser JI (2008) Resource availability influences the diversity of a functional group of heterotrophic soil bacteria. Environ Microbiol 10(9):2245–2256. doi:10.1111/j.1462-2920.2008.01647.x
Lee TD, Barrett JP, Hartman B (2005) Elevation, substrate, and the potential for climate-induced tree migration in the White Mountains, New Hampshire, USA. For Ecol Manage 212(1–3):75–91. doi:10.1016/j.foreco.2005.03.007
Loreau M (2001) Microbial diversity, producer-decomposer interactions and ecosystem processes: a theoretical model. Proc R Soc Lond Ser B-Biol Sci 268(1464):303–309. doi:10.1098/rspb.2000.1366
Martiny JBH, Bohannan BJM, Brown JH, Colwell RK, Fuhrman JA, Green JL, Horner-Devine MC, Kane M, Krumins JA, Kuske CR, Morin PJ, Naeem S, Ovreas L, Reysenbach AL, Smith VH, Staley JT (2006) Microbial biogeography: putting microorganisms on the map. Nat Rev Microbiol 4(2):102–112. doi:10.1038/nrmicro1341
McKenney DW, Pedlar JH, Lawrence K, Campbell K, Hutchinson MF (2007) Potential impacts of climate change on the distribution of North American trees. Bioscience 57(11):939–948. doi:10.1641/b571106
McLachlan JS, Clark JS, Manos PS (2005) Molecular indicators of tree migration capacity under rapid climate change. Ecology 86(8):2088–2098
Meentemeyer V (1978) Macroclimate and lignin control of litter decomposition rates. Ecology 59(3):465–472
Milcu A, Manning P (2011) All size classes of soil fauna and litter quality control the acceleration of litter decay in its home environment. Oikos 120(9):1366–1370. doi:10.1111/j.1600-0706.2010.19418.x
Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Brandt LA, Hart SC, Fasth B (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315(5810):361–364. doi:10.1126/science.1134853
Perala DA, Alban DH (1982) Rates of forest floor decomposition and nutrient turnover in aspen, pine and spruce stands on two different soils. Res Pap NC-227. US Department of Agriculture, Forest Service, North Central Forest Experimental Station, St Paul, p 5
Prasad AM, Iverson LR, Matthews S, Peters M (2007-ongoing) A Climate Change Atlas for 134 forest tree species of the Eastern United States [database]. Northern Research Station, USDA Forest Service, Delaware, Ohio. http://www.nrs.fs.fed.us/atlas/tree
Ramette A, Tiedje JM (2007) Multiscale responses of microbial life to spatial distance and environmental heterogeneity in a patchy ecosystem. Proc Natl Acad Sci U S A 104(8):2761–2766. doi:10.1073/pnas.0610671104
Rouifed S, Handa IT, David JF, Hattenschwiler S (2010) The importance of biotic factors in predicting global change effects on decomposition of temperate forest leaf litter. Oecologia 163(1):247–256. doi:10.1007/s00442-009-1528-1
Schimel JP, Hattenschwiler S (2007) Nitrogen transfer between decomposing leaves of different N status. Soil Biol Biochem 39(7):1428–1436. doi:10.1016/j.soilbio.2006.12.037
Schimel J, Schaeffer SM (2012) Microbial control over carbon cycling in soil. Frontiers Microbiol 3. doi:10.3389/fmicb.2012.00348
Smith VC, Bradford MA (2003) Do non-additive effects on decomposition in litter-mix experiments result from differences in resource quality between litters? Oikos 102(2):235–242
Strickland MS, Lauber C, Fierer N, Bradford MA (2009a) Testing the functional significance of microbial community composition. Ecology 90(2):441–451
Strickland MS, Osburn E, Lauber C, Fierer N, Bradford MA (2009b) Litter quality is in the eye of the beholder: initial decomposition rates as a function of inoculum characteristics. Funct Ecol 23(3):627–636. doi:10.1111/j.1365-2435.2008.01515.x
Tang GP, Beckage B, Smith B (2012) The potential transient dynamics of forests in New England under historical and projected future climate change. Clim Change 114(2):357–377. doi:10.1007/s10584-012-0404-x
Team RDC (2009) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
van der Heijden MGA, Bardgett RD, van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11(3):296–310. doi:10.1111/j.1461-0248.2007.01139.x
Wall DH, Bradford MA, St John MG, Trofymow JA, Behan-Pelletier V, Bignell DDE, Dangerfield JM, Parton WJ, Rusek J, Voigt W, Wolters V, Gardel HZ, Ayuke FO, Bashford R, Beljakova OI, Bohlen PJ, Brauman A, Flemming S, Henschel JR, Johnson DL, Jones TH, Kovarova M, Kranabetter JM, Kutny L, Lin KC, Maryati M, Masse D, Pokarzhevskii A, Rahman H, Sabara MG, Salamon JA, Swift MJ, Varela A, Vasconcelos HL, White D, Zou XM (2008) Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Glob Change Biol 14(11):2661–2677. doi:10.1111/j.1365-2486.2008.01672.x
Wallenstein MD, Hess AM, Lewis MR, Steltzerae H, Ayres E (2010) Decomposition of aspen leaf litter results in unique metabolomes when decomposed under different tree species. Soil Biol Biochem 42(3):484–490. doi:10.1016/j.soilbio.2009.12.001
Wertz S, Degrange V, Prosser JI, Poly F, Commeaux C, Freitag T, Guillaumaud N, Le Roux X (2006) Maintenance of soil functioning following erosion of microbial diversity. Environ Microbiol 8(12):2162–2169. doi:10.1111/j.1462-2920.2006.01098.x
Wolters V, Silver WL, Bignell DE, Coleman DC, Lavelle P, Van der Putten WH, De Ruiter P, Rusek J, Wall DH, Wardle DA, Brussaard L, Dangerfield JM, Brown VK, Giller KE, Hooper DU, Sala O, Tiedje J, Van Veen JA (2000) Effects of global changes on above- and belowground biodiversity in terrestrial ecosystems: implications for ecosystem functioning. Bioscience 50(12):1089–1098
Acknowledgments
This research was funded by the U.S. Forest Service through a cooperative agreement to MAB and JK, the U.S. Environmental Protection Agency through a Science to Achieve Results (STAR) Fellowship to ADK, and the U.S. National Science Foundation through the Coweeta Long Term Ecological Research (LTER) Program. We thank the National Park Service for granting us a research permit for the Blue Ridge Parkway National Park.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alfonso Escudero.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 112 kb)
Rights and permissions
About this article
Cite this article
Keiser, A.D., Knoepp, J.D. & Bradford, M.A. Microbial communities may modify how litter quality affects potential decomposition rates as tree species migrate. Plant Soil 372, 167–176 (2013). https://doi.org/10.1007/s11104-013-1730-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1730-0