Abstract
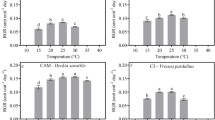
Among various epiphytic ferns found in the Brazilian Atlantic Forest, we studied Vittaria lineata (L.) Smith (Polypodiopsida, Pteridaceae). Anatomical characterization of the leaf was carried out by light microscopy, fluorescence microscopy, and scanning electron microscopy. V. lineata possesses succulent leaves with two longitudinal furrows on the abaxial surface. We observed abundant stomata inside the furrows, glandular trichomes, paraphises, and sporangia. We examined malate concentrations in leaves, relative water content (RWC), photosynthetic pigments, and chlorophyll (Chl) a fluorescence in control, water-deficient, and abscisic acid (ABA)-treated plants. Plants subjected to drought stress (DS) and treated by exogenous ABA showed significant increase in the malate concentration, demonstrating nocturnal acidification. These findings suggest that V. lineata could change its mode of carbon fixation from C3 to the CAM pathway in response to drought. No significant changes in RWC were observed among treatments. Moreover, although plants subjected to stress treatments showed a significant decline in the contents of Chl a and b, the concentrations of carotenoids were stable. Photosynthetic parameters obtained from rapid light curves showed a significant decrease after DS and ABA treatments.
Similar content being viewed by others
Abbreviations
- A :
-
scaling constant for the height of the light curve
- ABA:
-
abscisic acid
- AL:
-
actinic light
- Car:
-
carotenoids
- DM:
-
dry mass
- DS:
-
drought stress
- ETR:
-
electron transport rate
- ETRmax :
-
maximum electron transport rate
- FM:
-
fresh mass
- F0 :
-
minimum fluorescence
- Fm :
-
maximum fluorescence
- Fv :
-
variable fluorescence
- Fm′:
-
minimum fluorescence of a light-adapted leaf
- F′:
-
fluorescence yield of a light-adapted leaf
- FLM:
-
fluorescence microscopy
- Fv/Fm :
-
maximum photochemical efficiency of PSII
- I:
-
irradiance
- Iopt :
-
optimal irradiance for the saturation of photosynthesis
- kw :
-
scaling constant for the x-axis of the light curve
- LM:
-
light microscopy
- OAA:
-
oxaloacetic acid
- PAM:
-
pulse-amplitude modulation
- P :
-
photosynthesis
- PEG:
-
polyethylene glycol
- PEPC:
-
phosphoenolpyruvate carboxylase
- RLC:
-
rapid light curve
- RWC:
-
relative water content
- SEM:
-
scanning electron microscopy
- SL:
-
saturating light pulses
- TM:
-
turgid mass
- ϕPSII :
-
actual photochemical efficiency of PSII
References
Ashraf, M.Y., Azmi, A.R., Khan, A.H., Ala, S.A.: Effect of water stress on total phenols, peroxidase activity and chlorophyll content in wheat (Triticum aestivum L.). — Acta Physiol. Plant. 16: 185–191, 1994.
Barrs, H.D., Weatherley, P.E.: A re-examination of the relative turgidity technique for estimating water deficits in leaves. — Aust. J. Biol. Sci. 15: 413–428, 1962.
Bennet, B.C.: Patchiness, diversity and abundance relationships of vascular epiphytes. — Selbyana 9: 70–75, 1986.
Benzing, D.H.: The vegetative basis of vascular epiphytism. — Selbyana 9: 23–43, 1986.
Benzing, D.H.: Vascular Epiphytes. Pp. 376. Cambridge Univ. Press, Cambridge 1990.
Bjorkman, O., Demmig, B.: Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77-K among vascular plants of diverse origins. — Planta 170: 489–504, 1987.
Bukatsch, F.: [Observations of double staining Astra bluesafranin.] — Mikrokosmos 61: 255, 1972. [In German]
Brodribb, T.J., McAdam, S.A.M.: Passive origins of stomata control in vascular plants. — Science 331: 582–585, 2011.
Carter, J.P., Martin, C.E.: The occurrence of Crassulacean acid metabolism among epiphytes in a high-rainfall region of Costa Rica. — Selbyana 15: 104–106, 1994.
Chu, C., Dai, Z.Y., Ku, M. S. B., Edwards, G. E.: Induction of Crassulacean acid metabolism in the facultative halophyte Mesembryanthemum crystallinum by abscisic acid. — Plant. Physiol. 93: 1253–1260, 1990.
Costa, A. F.: [Pharmacognosy. Vol. 2.] Pp. 755. Fundação Calouste Gulbenkian, Lisboa 1982. [In Portuguese]
Constable, J. V. H., Grace, J. B., Longstreth, D. J.: High carbon dioxide concentrations in aerenchyma of Typha latifolia. — Am. J. Bot. 79: 415–418. 1992.
Cushman, J. C., Borland, A. M.: Induction of Crassulacean acid metabolism by water limitation. — Plant Cell Environ. 25: 295–310, 2002.
Drew, M. C., He, C.J., Morgan, P. W.: Programmed cell death and aerenchyma formation in roots. — Trends Plant Sci. 5: 123–127, 2000.
Freschi, L., Rodrigues, M. A., Tiné, M. A. S., Mercier, H.: Correlation between citric acid and nitrate metabolisms during CAM cycle in the atmospheric bromeliad Tillandsia pohliana. — J. Plant Physiol. 167: 1577–1583, 2010.
Genty, B., Briantais, J. M., Baker, N. R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. — Biochim. Biophys. Acta. 900: 87–92, 1989.
Gerlach, D.: [Botanical Microtechnology: An Introduction.] Pp. 311. Georg Thieme Verlag, Stuttgart 1984. [In German]
Gloag, R. S., Ritchie, R. J., Chen, M. et al.: Chromatic photoacclimation, photosynthetic electron transport and oxygen evolution in the chlorophyll d-containing oxyphotobacterium Acaryochloris marina. — Biochim. Biophys. Acta 1767: 127–135, 2007.
Griffiths, H., Ong, B. L., Avadhani, P. N., Goh, C. J.: Recycling of respiratory CO2 during Crassulacean acid metabolism: alleviation of photoinhibition in Pyrrosia piloselloides. — Planta 179: 115–122, 1989.
Hew, C. S., Wong, Y. S., Photosynthesis and respiration of ferns in relation to their habitat. — Am. Fern J. 64: 40–48, 1974.
Hietz, P., Briones, O.: Correlation between water relations and within-canopy distribution of epiphytic ferns in a Mexican cloud forest. — Oecologia 114: 305–316, 1998.
Hoagland, D. R., Arnon D.I.: The water culture method for growing plants without soil. — Calif. Exp. Stn. Circ. 347: 1–39, 1938.
Horton, R. F.: Stomatal opening: the role of abscisic acid. — Can. J. Bot. 49: 583–585, 1971.
Jaing, Y., Yang, W. Y., Jiang, X., Qiaoyon, C.: Active oxygen demand and effect on chlorophyll degradation in rice seedling under osmotic stress. — Acta Bot. Sin. 36: 289–295, 1994.
Jensen, W. A.: Botanical Histochemistry: Principles and Practice. Pp. 408. W. H. Freeman & Co, San Francisco 1962.
Johansen, D. A.: Plant Microtechnique. Pp. 523. McGraw Hill Book, New York 1940.
Kraus, J.E, Arduin, M.: [Basic Manual of Methods in Plant Morphology.] Pp. 198. Editora Universidade Rural. Seropédica, 1997. [In Portuguese]
Kluge, M., Avadhani, P. N., Goh, C. J.: Gas exchange and water relations in epiphytic tropical ferns. — In: Lüttge U. (ed.): Vascular Plants as Epiphytes. Ecological Studies 76. Pp. 87–109. Spriger-Verlag, Berlin 1989.
Kress, W. J.: A symposium: the biology of tropical epiphytes. — Selbyana 9: 1–22, 1986.
Lichtenthaler, H. K.: Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. — Method. Enzymol. 148: 350–382, 1987.
Lüttge, U.: The role of crassulacean acid metabolism (CAM) in the adaptation of plants to salinity. — New Phytol. 125: 59–71, 1993.
Madison, M.: Vascular epiphytes: their systematic occurrence and salient features. — Selbyana 2: 1–13, 1977.
Martin, S. L., Davis, R., Protti, P. et al.: The occurrence of crassulacean acid metabolism in epiphytic ferns, with an emphasis on the Vittariaceae. — Int. J. Plant Sci. 166: 623–630, 2005.
Maxwell, K., Johnson, G. N.: Chlorophyll fluorescence: A practical guide. — J. Exp. Bot. 51: 659–668, 2000.
McAdam, S. A. M., Brodribb, T. J.: Stomatal innovation and the rise of seed plants. — Ecol. Lett. 15: 1–8, 2012.
Möllering, H.: L(-) malate. — In: Bergmeyer, H.U. (ed.): Methods of Enzymatic Analysis. Pp. 39–47, Vol 7. VHC Verlagsgesellschaft, Weinheim 1985.
Neales, T. F, Hew, C. S.: Two types of carbon fixation in tropical orchids. — Planta 23: 303–306, 1975.
Nieder, J. Prosperí, J., Michaloud, G.: Epiphytes and their contribution to canopy diversity. — Plant Ecol. 153: 51–63, 2001.
Nimmo, H. G.: The regulation of phosphoenolpyruvate carboxylase in CAM plants. — Trends Plant Sci. 5: 75–80, 2000.
N’soukpoé-Kossi, C.N., Ivanov, A.G., Veeranjaneyulu, K., Leblanc, R. M. Protective action of abscisic acid against the inhibition of photosynthesis of barley leaves by bisulphite. — Photosynthetica 36: 51–60, 1999.
O’Brien, T. P., Feder, N., McCully, M.: Polychromatic staining of plant cell walls by toluidine blue. — Protoplasma 59: 368–373, 1965.
Pedersen, O., Sand-Jensen, K.: Adaptations of submerged Lobelia dortmanna to aerial life form: morphology, carbon sources, and oxygen dynamics. — Oikos 65: 89–96, 1992.
Peltzer, D., Dreyer, E., Polle, A.: Differential temperature dependencies of antioxidative enzymes in two contrasting species: Fagus sylvatica and Coleus blumei. — Plant Physiol. Bioch. 40: 141–150, 2002.
Pinhero, R. G., Rao, M. V., Paliyath, G. et al.: Changes in activities of antioxidant enzymes and their relationship to genetic and paclobutrazol-induced chilling tolerance of maize seedlings. — Plant Physiol. 114: 695–704, 1997.
Prado, J.: [Reviews and monographs as a basis for analysis of diversity, as we know about our flora.] — In: Jardim, M. A. G., Bastos, M. N. C., Santos, J. U. M. (ed.): [Challenges of Brazilian Botany in the new Millennium: Inventory, Systematic and Conservation of the Plant Diversity.] Pp. 78–79. MPEG/UFRA/EMBRAPA, Belém 2003. [In Portuguese]
Pryer, K. M., Schuettpelz, E., Wolf, P. G. et al.: Phylogeny and evolution of ferns (Monilophytes) with a focus on the early leptosporangiate divergences. — Am. J. Bot. 91: 1582–1598, 2004.
Ralph, P. J., Gademann, R.: Rapid light curves: a powerful tool to assess photosynthetic activity. — Aquat. Bot. 82: 222–237, 2005.
Rathinasabapathi, B.: Ferns represent untapped biodiversity for improving crops for enviromental stress tolerance. — New Phytol. 172: 385–390, 2006.
Ravensberg, W. J., Hennipman, E.: The Pyrrosia species formerly referred to as Drymoglossum and Saxiglossum. — Leiden Bot. Ser. 9: 281–310, 1986.
Reddy, A. R., Chaitanya, K. V., Vivekanandan, M.: Droughtinduced responses of photosynthesis and antioxidant metabolism in higher plants. — J. Plant Physiol. 161: 1189–1202, 2004.
Ritchie, R. J.: Fitting light saturation curves measured using modulated fluorometry. — Photosynth. Res. 96: 201–215, 2008.
Runcie, J. W., Durako, M. J.: Among-shoot variability and leafspecific absorptance characteristics affect diel estimates of in situ electron transport of Posidonia australis. — Aquat. Bot. 80: 209–220, 2004.
Rut, G., Krupa, J., Miszalski, Z. et al.: Crassulacean acid metabolism in the epiphytic fern Platycerium bifurcatum. — Photosynthetica 46: 156–160, 2008.
Ruzin, S. E.: Plant Microtechnique and Microscopy. Pp. 322–322. Oxford University Press, New York 1999.
Schreiber, U.: Pulse-amplitude (PAM) fluorometry and saturation pulse method. — In: Papageorgiou, G. and Govindjee (ed.): Chlorophyll a Fluorescence: a Signature of Photosynthesis. Advances in Photosynthesis and Respiration Series. Pp. 279–319. Kluwer Academic Publishers, Dordrecht 1994.
Smith, A. R., Pryer, K. M., Schuettpelz, E., Korall, P., Schneider, H., Wolf, P. G. A classification for extant ferns. — Taxon 55: 705–731, 2006.
Sinclair, R.: Water relations of tropical epiphytes. III. Evidence for crassulacean acid metabolism. — J. Exp. Bot. 35: 1–7, 1984.
Tausz, M., Hietz, P., Briones, O.: The significance of carotenoids and tocopherols in photoprotection of seven epiphytic fern species of a Mexican cloud forest. — Aust. J. Plant Physiol. 28: 775–783, 2001.
Tryon, R. M., Tryon, A.F.: Ferns and Allied Plants with Special Reference to Tropical America. Pp. 857. Springer-Verlag, New York 1982.
Uphof, J. C. T.: Physiological anatomy of xerophytic Selaginellas. — New Phytol. 19: 101–131, 1920.
White, A. J., Critchley, C.: Rapid light curves: a new fluorescence method to assess the state of the photosynthetic apparatus. — Photosynth. Res. 59: 63–72, 1999.
Winter, K., Wallace, B. J., Stocker, G. C., Roksandic, Z.: Crassulacean acid metabolism in Australian vascular epiphytes and some related species. — Oecologia 57: 129–141, 1983.
Wong, S. C., Hew, C. S.: Diffusive resistance, titratable acidity, and CO2 fixation in two tropical epiphytic ferns. — Am. Fern J. 66: 121–124, 1976.
Zar, J. H. Biostatistical Analysis. Pp. 718. Prentice Hall, New Jersey 1996.
Zotz, G. How prevalent is crassulacean acid metabolism among vascular epiphytes? — Oecologia 138: 184–192, 2004.
Zotz, G., Ziegler, H. The occurrence of crassulacean acid metabolism among vascular epiphytes from Central Panama. — New Phytol. 137: 223–229, 1997.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgments: The first authors would like to acknowledge CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for the scholarship (Rede em Epífitas de Mata Atlântica: Sistemática, Ecologia E Conservação, CAPES, PNADB, 2009). Á.M. Randi and M. Santos would like to acknowledge CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the research grants.
Rights and permissions
About this article
Cite this article
Minardi, B.D., Voytena, A.P.L., Santos, M. et al. Water stress and abscisic acid treatments induce the CAM pathway in the epiphytic fern Vittaria lineata (L.) Smith. Photosynthetica 52, 404–412 (2014). https://doi.org/10.1007/s11099-014-0047-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-014-0047-4