Abstract
Purpose
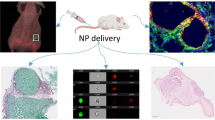
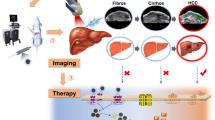
To develop polymer nanoassemblies (PNAs) modified with halofluorochromic dyes to allow for the detection of liver metastatic colorectal cancer (CRC) to improve therapeutic outcomes.
Methods
We combine experimental and computational approaches to evaluate macroscopic and microscopic PNA distributions in patient-derived xenograft primary and orthotropic liver metastatic CRC tumors. Halofluorochromic and non-halofluorochromic PNAs (hfPNAs and n-hfPNAs) were prepared from poly(ethylene glycol), fluorescent dyes (Nile blue, Alexa546, and IR820), and hydrophobic groups (palmitate), all of which were covalently tethered to a cationic polymer scaffold [poly(ethylene imine) or poly(lysine)] forming particles with an average diameter < 30 nm.
Results
Dye-conjugated PNAs showed no aggregation under opsonizing conditions for 24 h and displayed low tissue diffusion and cellular uptake. Both hfPNAs and n-hfPNAs accumulated in primary and liver metastatic CRC tumors within 12 h post intravenous injection. In comparison to n-hfPNAs, hfPNAs fluoresced strongly only in the acidic tumor microenvironment (pH < 7.0) and distinguished small metastatic CRC tumors from healthy liver stroma. Computational simulations revealed that PNAs would steadily accumulate mainly in acidic (hypoxic) interstitium of metastatic tumors, independently of the vascularization degree of the tissue surrounding the lesions.
Conclusion
The combined experimental and computational data confirms that hfPNAs detecting acidic tumor tissue can be used to identify small liver metastatic CRC tumors with improved accuracy.













Similar content being viewed by others
Change history
16 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11095-021-03074-0
Abbreviations
- CRC:
-
Colorectal cancer
- hfPNA:
-
Halofluorochromic PNA
- hfPNA#/NB:
-
hfPNA with PEG-PEI scaffold labeled with NB dye
- hfPNA/NB:
-
hfPNA labeled with NB dye
- NB:
-
Nile blue
- n-hfPNA:
-
Non-halofluorochromic PNA
- n-hfPNA/Alexa546:
-
n-hfPNA labeled with Alexa546 dye
- n-hfPNA/IR820:
-
n-hfPNA labeled with IR820 dye
- PAL:
-
Palmitatic acid
- PDX:
-
Patient-derived xenograft
- PEG:
-
Poly(ethylene glycol)
- PEI:
-
Poly(ethylene imine)
- PLL:
-
Poly(lysine)
- PNA:
-
Polymer nanoassembly
References
Robinson PJ. The early detection of liver metastases. Cancer Imaging. 2002;2(2):1–3.
Spano D, Heck C, De Antonellis P, Christofori G, Zollo M. Molecular networks that regulate cancer metastasis. Semin Cancer Biol. 2012;22(3):234–49.
Hugen N, van de Velde CJH, de Wilt JHW, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014;25(3):651–7.
Van Cutsem E, Verheul HM, Flamen P, Rougier P, Beets-Tan R, Glynne-Jones R, et al. Imaging in colorectal cancer: progress and challenges for the clinicians. Cancers (Basel). 2016;8(9):81.
Kamiya M, Urano Y. Rapid and sensitive fluorescent imaging of tiny tumors in vivo and in clinical specimens. Curr Opin Chem Biol. 2016;33:9–15.
Maffione AM, Lopci E, Bluemel C, Giammarile F, Herrmann K, Rubello D. Diagnostic accuracy and impact on management of (18)F-FDG PET and PET/CT in colorectal liver metastasis: a meta-analysis and systematic review. Eur J Nucl Med Mol Imaging. 2015;42(1):152–63.
Barnes KD, Shafirstein G, Webber JS, Koonce NA, Harris Z, Griffin RJ. Hyperthermia-enhanced indocyanine green delivery for laser-induced thermal ablation of carcinomas. Int J Hyperth. 2013;29(5):474–9.
Wu L, Fang S, Shi S, Deng J, Liu B, Cai L. Hybrid polypeptide micelles loading indocyanine green for tumor imaging and photothermal effect study. Biomacromolecules. 2013;14(9):3027–33.
Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumors depends on size. Nat Nanotechnol. 2011;6(12):815–23.
Mittapalli RK, Adkins CE, Bohn KA, Mohammad AS, Lockman JA, Lockman PR. Quantitative fluorescent microscopy to measure vascular pore sizes in primary and metastatic brain tumors. Cancer Res. 2016;77(2):238–46.
Ernsting MJ, Murakami M, Roy A, Li S-D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J Control Release. 2013;172(3):782–94.
Rannard S, Owen A. Nanomedicine: not a case of “one size fits all”. Nano Today. 2009;4(5):382–4.
Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63(3):131–5.
Svenson S. What nanomedicine in the clinic right now really forms nanoparticles? Nanomed Nanobiotech. 2014;6(2):125–35.
Zamboni WC, Torchilin V, Patri AK, Hrkach J, Stern S, Lee R, et al. Best practices in cancer nanotechnology: perspective from NCI nanotechnology alliance. Clin Cancer Res. 2012;18(12):3229–41.
Blanco E, Hsiao A, Mann Aman P, Landry Matthew G, Meric-Bernstam F, Ferrari M. Nanomedicine in cancer therapy: innovative trends and prospects. Cancer Sci. 2011;102(7):1247–52.
Kim BYS, Rutka JT, Chan WCW. Current concepts: nanomedicine. New Engl J Med. 2010;363(25):2434–43.
Crist RM, Grossman JH, Patri AK, Stern ST, Dobrovolskaia MA, Adiseshaiah PP, et al. Common pitfalls in nanotechnology: lessons learned from NCI's nanotechnology characterization laboratory. Integr Biol. 2013;5(1):66–73.
Venditto VJ, Szoka FC. Cancer nanomedicines: so many papers and so few drugs! Adv Drug Deliv Rev. 2013;65(1):80–8.
Moghimi SM, Farhangrazi ZS. Just so stories: the random acts of anti-cancer nanomedicine performance. Nanomedicine. 2014;10(8):1661–6.
Lee HJ, Bae Y. Pharmaceutical differences between block copolymer self-assembled and cross-linked nanoassemblies as carriers for tunable drug release. Pharm Res. 2013;30(2):478–88.
Dickerson M, Bae Y. Block copolymer nanoassemblies for photodynamic therapy and diagnosis. Ther Deliv. 2013;4(11):1431–41.
Lee HJ, Bae Y. Cross-linked nanoassemblies from poly(ethylene glycol)-poly(aspartate) block copolymers as stable supramolecular templates for particulate drug delivery. Biomacromolecules. 2011;12:2686–96.
Lee HJ, Ponta A, Bae Y. Polymer nanoassemblies for cancer treatment and imaging. Ther Deliv. 2010;1(6):803–17.
Bae Y. Drug delivery systems using polymer nanoassemblies for cancer treatment. Ther Deliv. 2010;1:361–3.
Reichel D, Lee MJ, Lee W, Kim KB, Bae Y. Tethered polymer Nanoassemblies for sustained carfilzomib release and prolonged suppression of proteasome activity. Ther Deliv. 2016;7(10):665–81.
Reichel D, Bae Y. Comparison of dialysis-and Solvatofluorochromism-based methods to determine drug release rates from polymer Nanoassemblies. Pharm Res. 2017;34(2):394–407.
Reichel D, Rychahou P, Bae Y. Polymer nanoassemblies with solvato-and halo-fluorochromism for drug release monitoring and metastasis imaging. Ther Deliv. 2015;6(10):1221–37.
Ao L, Reichel D, Hu D, Jeong H-Y, Kim KB, Bae Y, et al. Polymer micelle formulations of proteasome inhibitor carfilzomib for improved metabolic stability and anti-cancer efficacy in human multiple myeloma and lung cancer cell lines. J Pharmacol Exp Ther. 2015;355:168–73.
Rheiner S, Rychahou P, Bae Y. Effects of the lipophilic Core of polymer Nanoassemblies on intracellular delivery and transfection of siRNA. AIMS Biophysics. 2015;2(3):284–302.
Rheiner S, Bae Y. Increased poly(ethylene glycol) density decreases transfection efficacy of siRNA/poly(ethylene imine) complexes. AIMS Bioeng. 2016;3(4):454–67.
Dickerson M, Howerton B, Bae Y, Glazer E. Light-sensitive ruthenium complex-loaded cross-linked polymeric nanoassemblies for the treatment of cancer. J Mater Chem B Mater Biol Med. 2016;4:394–408.
Dickerson M, Winquist N, Bae Y. Photo-inducible cross-linked nanoassemblies for controlled drug delivery. Pharm Res. 2013;31:1254–63.
Dan M, Scott DF, Hardy PA, Wydra RJ, Hilt JZ, Yokel RA, et al. Block copolymer cross-linked nanoassemblies improve particle stability and biocompatibility of superparamagnetic iron oxide nanoparticles. Pharm Res. 2013;30(2):552–61.
Curtis LT, Rychahou P, Bae Y, Frieboes HB. A computational/experimental assessment of antitumor activity of polymer nanoassemblies for ph-controlled drug delivery to primary and metastatic tumors. Pharm Res. 2016;33:2552–64.
Vazquez A, Liu J, Zhou Y, Oltvai ZN. Catabolic efficiency of aerobic glycolysis: the Warburg effect revisited. BMC Syst Biol. 2010;4:58.
Vander Heiden MG, Thompson CB, Cantley LC. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33.
van de Ven AL, Abdollahi B, Martinez CJ, Burey LA, Landis MD, Chang JC, et al. Modeling of nanotherapeutics delivery based on tumor perfusion. New J Phys. 2013;15(5):55004.
Wu M, Frieboes HB, Chaplain MAJ, McDougall SR, Cristini V, Lowengrub JS. The effect of interstitial pressure on therapeutic agent transport: coupling with the tumor blood and lymphatic vascular systems. J Theor Biol. 2014;355:194–207.
Wu M, Frieboes HB, McDougall SR, Chaplain MAJ, Cristini V, Lowengrub J. The effect of interstitial pressure on tumor growth: coupling with the blood and lymphatic vascular systems. J Theor Biol. 2013;320:131–51.
Zaytseva YY, Elliott VA, Rychahou P, Mustain WC, Kim JT, Valentino J, et al. Cancer cell-associated fatty acid synthase activates endothelial cells and promotes angiogenesis in colorectal cancer. Carcinogenesis. 2014;35(6):1341–51.
Elliott VA, Rychahou P, Zaytseva YY, Evers BM. Activation of c-met and upregulation of CD44 expression are associated with the metastatic phenotype in the colorectal cancer liver metastasis model. PLoS One. 2014;9(5):e97432.
Zaytseva YY, Rychahou PG, Gulhati P, Elliott VA, Mustain WC, O'Connor K, et al. Inhibition of fatty acid synthase attenuates CD44-associated signaling and reduces metastasis in colorectal cancer. Cancer Res. 2012;72(6):1504–17.
Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71(9):3246–56.
Rychahou PG, Kang J, Gulhati P, Doan HQ, Chen LA, Xiao SY, et al. Akt2 overexpression plays a critical role in the establishment of colorectal cancer metastasis. PNAS. 2008;105(51):20315–20.
Reichel D, Bae Y. Comparison of dialysis- and Solvatofluorochromism-based methods to determine drug release rates from polymer Nanoassemblies. Pharm Res 2017;34(2):394–407.
Chaudhari KR, Ukawala M, Manjappa AS, Kumar A, Mundada PK, Mishra AK, et al. Opsonization, biodistribution, cellular uptake and apoptosis study of PEGylated PBCA nanoparticle as potential drug delivery carrier. Pharm Res. 2012;29(1):53–68.
Zhang M, Liu Y-Q, Ye B-C. Colorimetric assay for sulfate using positively-charged gold nanoparticles and its application for real-time monitoring of redox process. Analyst. 2011;136(21):4558–62.
Rausch K, Reuter A, Fischer K, Schmidt M. Evaluation of nanoparticle aggregation in human blood serum. Biomacromolecules. 2010;11(11):2836–9.
Opitz AW, Czymmek KJ, Wickstrom E, Wagner NJ. Uptake, efflux, and mass transfer coefficient of fluorescent PAMAM dendrimers into pancreatic cancer cells. Biochim Biophys Acta. 2013;1828(2):294–301.
van de Ven AL, Wu M, Lowengrub J, McDougall SR, Chaplain MA, Cristini V, et al. Integrated intravital microscopy and mathematical modeling to optimize nanotherapeutics delivery to tumors. AIP Adv. 2012;2(1):11208.
Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–51.
Kekelidze M, D'Errico L, Pansini M, Tyndall A, Hohmann J. Colorectal cancer: current imaging methods and future perspectives for the diagnosis, staging and therapeutic response evaluation. World J Gastroenterol. 2013;19(46):8502–14.
Terranova N, Girard P, Klinkhardt U, Munafo A. Resistance development: a major piece in the jigsaw puzzle of tumor size modeling. CPT Pharmacometrics Syst Pharmacol. 2015;4(6):320–3.
Etrych T, Lucas H, Janouskova O, Chytil P, Mueller T, Mader K. Fluorescence optical imaging in anticancer drug delivery. J Control Release. 2016;226:168–81.
Cao P, Ponta A, Kim JA, Bae Y. Block copolymer crosslinked nanoassemblies co-entrapping acridine yellow and doxorubicin for cancer theranostics. British J Pharm Res. 2013;3(4):523–35.
Shao D, Lu MM, Zhao YW, Zhang F, Tan YF, Zheng X, et al. The shape effect of magnetic mesoporous silica nanoparticles on endocytosis, biocompatibility and biodistribution. Acta Biomater. 2017;49:531–40.
Kumar R, Roy I, Ohulchanskky TY, Vathy LA, Bergey EJ, Sajjad M, et al. In vivo biodistribution and clearance studies using multimodal organically modified silica nanoparticles. ACS Nano. 2010;4(2):699–708.
Sindhwani S, Syed AM, Wilhelm S, Glancy DR, Chen YY, Dobosz M, et al. Three-dimensional optical mapping of nanoparticle distribution in intact tissues. ACS Nano. 2016;10(5):5468–78.
Verkman AS. Diffusion in the extracellular space in brain and tumors. Phys Biol. 2013;10(4):045003.
Shrinivas P, Kasapis S, Tongdang T. Morphology and mechanical properties of bicontinuous gels of agarose and gelatin and the effect of added lipid phase. Langmuir. 2009;25(15):8763–73.
Tufto I, Lyng H, Rofstad EK. Interstitial fluid pressure, perfusion rate and oxygen tension in human melanoma xenografts. Br J Cancer Suppl. 1996;27:S252–5.
Watson KD, Lai CY, Qin S, Kruse DE, Lin YC, Seo JW, et al. Ultrasound increases nanoparticle delivery by reducing intratumoral pressure and increasing transport in epithelial and epithelial-mesenchymal transition tumors. Cancer Res. 2012;72(6):1485–93.
Durymanov MO, Rosenkranz AA, Sobolev AS. Current approaches for improving Intratumoral accumulation and distribution of nanomedicines. Theranostics. 2015;5(9):1007–20.
Beck-Broichsitter M, Nicolas J, Couvreur P. Design attributes of long-circulating polymeric drug delivery vehicles. Eur J Pharm Biopharm. 2015;97(Pt B):304–17.
Gao H, He Q. The interaction of nanoparticles with plasma proteins and the consequent influence on nanoparticles behavior. Expert Opin Drug Deliv. 2014;11(3):409–20.
Acknowledgements and Disclosures
This work was supported by the University of Kentucky Graduate School Allocated Year (GSAY) Fellowship (DR) and the National Institutes of Health grant R01CA195573 (BME and PR). HBF acknowledges partial support by the National Institutes of Health /National Cancer Institute (R15CA203605).
Author information
Authors and Affiliations
Corresponding author
Additional information
Piotr Rychahou, Hermann B. Frieboes, and Younsoo Bae share joint senior authorship.
Electronic Supplementary Material
ESM 1
(DOCX 1279 kb)
Rights and permissions
About this article
Cite this article
Reichel, D., Curtis, L.T., Ehlman, E. et al. Development of Halofluorochromic Polymer Nanoassemblies for the Potential Detection of Liver Metastatic Colorectal Cancer Tumors Using Experimental and Computational Approaches. Pharm Res 34, 2385–2402 (2017). https://doi.org/10.1007/s11095-017-2245-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-017-2245-9