Abstract
Purpose
We developed simulation and modeling methods to predict the in vivo pharmacokinetic profiles of acyclovir, following escalating oral doses of valacyclovir, in wildtype and Pept1 knockout mice. We also quantitated the contribution of specific intestinal segments in the absorption of valacyclovir in these mice.
Methods
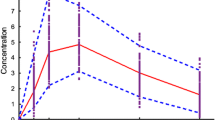
Simulations were conducted using a mechanistic advanced compartmental absorption and transit (ACAT) model implemented in GastroPlus™. Simulations were performed for 3 h post-dose in wildtype and Pept1 knockout mice following single oral doses of 10, 25, 50 and 100 nmol/g valacyclovir, and compared to experimentally observed plasma concentration-time profiles of acyclovir.
Results
Good fits were obtained in wildtype and Pept1 knockout mice. Valacyclovir was primarily absorbed from duodenum (42%) and jejunum (24%) of wildtype mice, with reduced uptake from ileum (3%) and caecum/colon (1%), for a total of 70% absorption. In contrast, the absorption of valacyclovir in Pept1 knockout mice was slow and sustained throughout the entire intestinal tract in which duodenum (4%), jejunum (14%), ileum (10%) and caecum/colon (12%) accounted for a total of 40% absorption.
Conclusion
The ACAT model bridged the gap between in situ and in vivo experimental findings, and facilitated our understanding of the complicated intestinal absorption processes of valacyclovir.







Similar content being viewed by others
Abbreviations
- ACAT:
-
Advanced compartmental absorption and transit
- AUC0–180 :
-
Area under the plasma concentration-time curve from time 0 to 180 min
- Cmax :
-
Peak plasma concentration
- GI:
-
Gastrointestinal
- Peff :
-
Intestinal permeability
- PEPT1:
-
Peptide Transporter 1
- PSA:
-
Parameter sensitivity analysis
References
Brandsch M, Knütter I, Bosse-Doenecke E. Pharmaceutical and pharmacological importance of peptide transporters. J Pharm Pharmacol. 2008;60:543–85.
Rubio-Aliaga I, Daniel H. Peptide transporters and their roles in physiological processes and drug disposition. Xenobiotica. 2008;38:1022–42.
Brandsch M. Transport of drugs by proton-coupled peptide transporters: pearls and pitfalls. Expert Opin Drug Metab Toxicol. 2009;5:887–905.
Brandsch M. Drug transport via the intestinal peptide transporter PepT1. Curr Opin Pharmacol. 2013;13:881–7.
Smith DE, Clémençon B, Hediger MA. Proton-coupled oligopeptide transporter family SLC15: physiological, pharmacological and pathological implications. Mol Asp Med. 2013;34:323–36.
Varma MV, Ambler CM, Ullah M, Rotter CJ, Sun H, Litchfield J, et al. Targeting intestinal transporters for optimizing oral drug absorption. Curr Drug Metab. 2010;11:730–42.
Dahan A, Khamis M, Agbaria R, Karaman R. Targeted prodrugs in oral drug delivery: the modern molecular biopharmaceutical approach. Expert Opin Drug Deliv. 2012;9:1001–13.
Zhang L, Zhang L, Luo T, Zhou J, Sun L, Xu Y. Synthesis and evaluation of a dipeptide-drug conjugate library as substrates for PEPT1. ACS Comb Sci. 2012;14:108–14.
Gupta D, Varghese Gupta S, Dahan A, Tsume Y, Hilfinger J, Lee KD, et al. Increasing oral absorption of polar neuraminidase inhibitors: a prodrug transporter approach applied to oseltamivir analogue. Mol Pharm. 2013;10:512–22.
Yang B, Smith DE. Significance of peptide transporter 1 in the intestinal permeability of valacyclovir in wild-type and PepT1 knockout mice. Drug Metab Dispos. 2013;41:608–14.
Yang B, Hu Y, Smith DE. Impact of peptide transporter 1 on the intestinal absorption and pharmacokinetics of valacyclovir after oral dose escalation in wild-type and PepT1 knockout mice. Drug Metab Dispos. 2013;41:1867–74.
Grass GM. Simulation models to predict oral drug absorption from in vitro data. Adv Drug Deliv Rev. 1997;23:199–219.
Huang W, Lee SL, Yu LX. Mechanistic approaches to predicting oral drug absorption. AAPS J. 2009;11:217–24.
Yu LX, Amidon GL. Saturable small intestinal drug absorption in humans: modeling and interpretation of cefatrizine data. Eur J Pharm Biopharm. 1998;45:199–203.
Yokoe J, Iwasaki N, Haruta S, Kadono K, Ogawara K, Higaki K, et al. Analysis and prediction of absorption behavior of colon-targeted prodrug in rats by GI-transit-absorption model. J Control Release. 2003;86:305–13.
Haddish-Berhane N, Farhadi A, Nyquist C, Haghighi K, Keshavarzian A. SIMDOT-AbMe: microphysiologically based simulation tool for quantitative prediction of systemic and local bioavailability of targeted oral delivery formulations. Drug Metab Dispos. 2009;37:608–18.
Hironaka T, Itokawa S, Ogawara KI, Higaki K, Kimura T. Quantitative evaluation of PEPT1 contribution to oral absorption of cephalexin in rats. Pharm Res. 2009;26:40–50.
Yu LX, Lipka E, Crison JR, Amidon G.L. Transport approaches to the biopharmaceutical design of oral drug delivery systems: prediction of intestinal absorption. Adv Drug Deliv Rev 1996;19:359–376.
Yu LX, Amidon GL. A compartmental absorption and transit model for estimating oral drug absorption. Int J Pharm. 1999;186:119–25.
Agoram B, Woltosz WS, Bolger MB. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv Drug Deliv Rev. 2001;50(Suppl 1):S41–67.
Tubic M, Wagner D, Spahn-Langguth H, Bolger MB, Langguth P. In silico modeling of non-linear drug absorption for the P-gp substrate talinolol and of consequences for the resulting pharmacodynamic effect. Pharm Res. 2006;23:1712–20.
Bolger MB, Lukacova V, Woltosz WS. Simulations of the nonlinear dose dependence for substrates of influx and efflux transporters in the human intestine. AAPS J. 2009;11:353–63.
Abuasal BS, Bolger MB, Walker DK, Kaddoumi A. In silico modeling for the nonlinear absorption kinetics of UK-343,664: a P-gp and CYP3A4 substrate. Mol Pharm. 2012;9:492–504.
Balimane P, Sinko P. Effect of ionization on the variable uptake of valacyclovir via the human intestinal peptide transporter (hPepT1) in CHO cells. Biopharm Drug Dispos. 2000;21:165–74.
Jappar D, Wu SP, Hu Y, Smith DE. Significance and regional dependency of peptide transporter (PEPT) 1 in the intestinal permeability of glycylsarcosine: in situ single-pass perfusion studies in wild-type and Pept1 knockout mice. Drug Metab Dispos. 2010;38:1740–6.
Yang B. Role of peptide transporter PEPT1 in the intestinal absorption and pharmacokinetics of the amino acid ester prodrug valacyclovir (doctoral dissertation). Ann Arbor: The University of Michigan; 2012.
de Miranda P, Krasny HC, Page DA, Elion GB. The disposition of acyclovir in different species. J Pharmacol Exp Ther. 1981;219:309–15.
Granero GE, Amidon GL. Stability of valacyclovir: implications for its oral bioavailability. Int J Pharm. 2006;317:14–8.
Takeda M, Khamdang S, Narikawa S, Kimura H, Kobayashi Y, Yamamoto T, et al. Human organic anion transporters and human organic cation transporters mediate renal antiviral transport. J Pharmacol Exp Ther. 2002;300:918–24.
Jappar D, Hu Y, Smith DE. Effect of dose escalation on the in vivo oral absorption and disposition of glycylsarcosine in wild-type and Pept1 knockout mice. Drug Metab Dispos. 2011;39:2250–7.
Weller S, Blum MR, Doucette M, Burnette T, Cederberg DM, de Miranda P, et al. Pharmacokinetics of the acyclovir pro-drug valaciclovir after escalating single- and multiple-dose administration to normal volunteers. Clin Pharmacol Ther. 1993;54:595–605.
Ormrod D. Goa K (2000) Valaciclovir: a review of its use in the management of herpes zoster. Drugs. 2000;59:1317–40.
Anand BS, Katragadda S, Mitra AK. Pharmacokinetics of novel dipeptide ester prodrugs of acyclovir after oral administration: intestinal absorption and liver metabolism. J Pharmacol Exp Ther. 2004;311:659–67.
Hu Y, Smith DE. Species differences in the pharmacokinetics of cefadroxil as determined in wildtype and humanized PepT1 mice. Biochem Pharmacol. 2016;107:81–90.
Hu Y, Xie Y, Wang Y, Chen X, Smith DE. Development and characterization of a novel mouse line humanized for the intestinal peptide transporter. Mol Pharm. 2014;11(10):3737–46.
Acknowledgments and Disclosures
This work was supported by Public Health Service grant R01GM115481 from the National Institute of General Medical Sciences (to D.E.S.). We would like to thank Dr. Michael B. Bolger (Simulations Plus, Inc., Lancaster, CA) for his suggestions with modeling and insightful comments on our findings.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, B., Smith, D.E. In Silico Absorption Analysis of Valacyclovir in Wildtype and Pept1 Knockout Mice Following Oral Dose Escalation. Pharm Res 34, 2349–2361 (2017). https://doi.org/10.1007/s11095-017-2242-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-017-2242-z