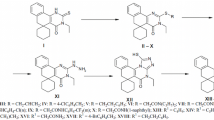
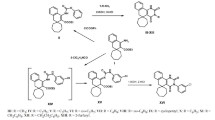
Amides that cyclized into 2-substituted 3H-spiro[benzo(h)quinazoline-5,1′-cyclohexane]-4(6H)-ones were prepared by reaction of 4′-amino-1′H-spiro[cyclohexane-1,2′-naphthalene]-3′-nitrile with carboxylic acid chlorides. Substitution of the benzoquinazolines by alcoholates, secondary amines, and thiolates was investigated. Thus,2-(3-chloropropyl)-3H-spiro[benzo[h]quinazoline-5,1′-cyclohexane]-4(6H)-one underwent intramolecular cyclization to form 10,11-dihydro-5H-spiro[benzo[h]pyrrolo[2,1-b]quinazoline-6,1′-cyclohexane]- 7(9H)-one whereas 2-chloromethyl-3H-spiro[benzo[h]quinazoline-5,1′-cyclohexane]-4(6H)-one formed substitution products. The influence of the synthesized compounds on brain monoamine oxidase activity was studied in vitro. It was found that the majority of the compounds inhibited deamination of 5-HT. The antitumor activity of the compounds was studied using two grafted murine tumor models, i.e., Ehrlich ascites carcinoma and sarcoma 180. Some of the investigated compounds suppressed tumor growth by 50 – 56%.
Similar content being viewed by others
References
A. I. Markosyan, S. V. Dilanyan, R. A. Kuroyan, et al., Khim.-farm. Zh., 29(4), 32 – 34 (1995); Pharm. Chem. J., 29(4), 259 – 269 (1995).
I. Sovadinova, L. Blaha, J. Janosek, K. Hilcherova, et al., Environ. Toxicol. Chem., 25(5), 1291 – 1297 (2008).
B. Van Treist and G. J. Peters, Oncology, 57(3), 179 – 194 (1999).
M. Hanlon and R. Ferone, Cancer Res., 56(14), 3301 – 3306 (1996).
A. I. Markosyan, S. A. Pogosyan, M. S. Safaryan, et al., Khim.-farm. Zh., 41(4), 16 – 18 (2006); Pharm. Chem. J., 41(4), 193 – 196 (2007).
A. I. Markosyan, S. A. Gabrielyan, G. A. Panosyan, et al., Khim.-farm. Zh., 42(2), 6 – 9 (2008).
A. I. Markosyan, M. G. Oganisyan, and R. A. Kuroyan, Khim. Geterotsikl. Soedin., No. 5, 658 – 661 (1992).
R. A. Kuroyan, A. I. Markosyan, A. Sh. Oganisyan, and M. G. Oganisyan, Arm. Khim. Zh., 42(8), 527 (1989).
G. N. Pershin, Methods of Experimental Chemotherapy [in Russian], Medgiz, Moscow (1971).
Z. P. Sof’ina, A. B. Syrkin, A. Goldin, et al., Experimental Assessment of Antitumor Compounds in the USSR and USA [in Russian], Meditsina, Moscow (1980).
R. R. Safrazbekyan and R. S. Sukasyan, Vopr. Med. Khim., 16, 623 – 628 (1970).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 48, No. 6, pp. 14 – 18, June, 2014.
Rights and permissions
About this article
Cite this article
Markosyan, A.I., Gabrielyan, S.A., Arsenyan, F.G. et al. Synthesis and Anti-Monoamine Oxidase and Antitumor Properties of Novel 3H-Spiro[Benzo[H] Quinazoline-5,1′-Cyclohexane]-4(6H)-One Derivatives. Pharm Chem J 48, 368–372 (2014). https://doi.org/10.1007/s11094-014-1112-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-014-1112-9