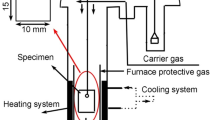
Abstract
The longer term oxidation behaviour of a low carbon, low silicon steel in 17H2O–N2 at 900°C was examined. The oxidation kinetics exhibited a multi-stage pattern with the first stage being initially linear and then transitioned to parabolic with a rate constant two orders of magnitude smaller than prediction. This was followed by an accelerated stage, and then a second parabolic stage with a rate constant much closer to that of iron and steel oxidation in air or oxygen. A single-phase wüstite layer was observed throughout the oxidation period. Using Wagner’s scaling equation, the effective oxygen potential (oxygen potential achieved at the outer surface of the wüstite layer) was calculated. During the first parabolic stage, this potential was extremely low, leading to a very low oxidation rate. It was then increased continuously through the accelerated oxidation stage to a much higher level at the second parabolic stage. The possible mechanism responsible for the observed trend of effective oxygen potential change and the corresponding trend of oxidation kinetics was discussed.







Similar content being viewed by others
References
R. Y. Chen and W. Y. D. Yuen, Metallurgical and Materials Transactions A 40A, 2009 (3091).
C. Wagner, Diffusion and High Temperature Oxidation of Metals, (Atom Movements, ASM, Cleveland, 1951), p. 153.
H. Yin, S. L. I. Chan, W. Y. D. Yuen and D. J. Young, Oxidation of Metals 77, 2012 (305).
H. Yin, W. Y. D. Yuen and D. J. Young, Materials and Corrosion 63, 2012 (869).
D. J. Young and H. Yin, Oxidation of Metals 79, 2013 (445).
C. Wagner, Z Physikal Chemistry B 21, 1933 (25–41).
C. Wagner, Z Physikal Chemistry B 32, 1936 (447–462).
D. Young, High Temperature Oxidation and Corrosion of Metals, (Elsevier, Amsterdam, 2008), pp. 93–96.
S. Mrowec, Defects and Diffusion in Solids—An Introduction, (Elsevier Scientific Publishing Company, Amsterdam, 1980), p. 200.
J. R. Manning, Diffusion and ionic conductivity: kinetic theory, Mass Transport in Oxides: Proceedings of a symposium held at Gaithersburg, Maryland, October 22–25, 1967, (National Bureau of Standards Special Publication 296, 1968), pp. 53–63
C. Wagner, Progress in Solid State Chemistry 10, (1), 1975 (3).
R. Y. Chen and W. Y. D. Yuen, Oxidation of Metals 59, 2003 (433).
R. Y. Chen and W. Y. D. Yuen, Oxidation of Metals 79, 2013 (679).
R. Y. Chen and W. Y. D. Yuen, Oxidation of Metals 70, 2008 (39).
L. Himmel, R. F. Mehl and C. E. Birchenall, Journal of Metals (Transactions AIME) 5, 1953 (827).
J. Paidassi, Acta Metallurgica 3, 1955 (447).
R. Y. Chen and W. Y. D. Yuen, Oxidation of Metals 53, 2000 (539).
K. Akiba, M. Ueda, K. Kawamura and T. Maruyama, Materials Transactions 49, 2008 (629).
R. Bredesen and P. Kofstad, Oxidation of Metals 34, 1990 (361).
R. Bredesen and P. Kofstad, Oxidation of Metals 35, 1991 (107).
R. Bredesen and P. Kofstad, Oxidation of Metals 36, 1991 (27).
W. W. Smeltzer, Acta Metallurgica 8, 1960 (377).
H. J. Grabke, K. J. Best and A. Gala, Werkst U Korrosion 21, 1970 (911).
H. J. Grabke, V. Leroy and H. Hiefhaus, ISIJ International 35, 1995 (95).
H. J. Grabke, Materiali in Technologije 40, (2), 2006 (39).
D. Fujita, T. Ohgi and T. Homma, Applied Surface Science 200, 2002 (55).
H. J. Grabke, E. M. Petersen and S. R. Srinivasan, Surface Science 67, 1977 (501).
L. S. Darken and R. W. Gurry, Journal of the American Chemical Society 67, 1945 (1398–1412).
L. S. Darken and R. W. Gurry, Journal of the American Chemical Society 68, 1946 (798–816).
E. R. Jette and F. Foote, Journal of Chemical Physics 1, 1933 (29).
F. Morin, Scripta Metallurgica 10, 1976 (553).
P. Vallet and P. Raccah, Mem. Sci. Rev. Metall. 62, (1), 1965 (1).
J. Nowotny and M. Rekas, Journal of the American Ceramic Society 72, (7), 1989 (1221).
S. Mrowec and A. Podgorecka, Journal of Materials Science 22, 1987 (4181).
P. Kofstad and A. Z. Hed, Journal of the Electrochemical Society 115, (1), 1968 (102).
P. Kofstad, Nonstoichiometry, Diffusion, and Electrical Conductivity in Binary Metal Oxides, (Wiley—Interscience, New York, 1972).
H.-J. Engell, Acta Metallurgica 6, 1958 (439).
W. K. Chen and N. L. Peterson, Journal of Physics and Chemistry of Solids 36, 1975 (1097).
G. Garnaud and R. A. Rapp, Oxidation of Metals 11, (4), 1977 (193).
R. M. Hazen, Reviews of Geophysics and Space Physics 22, (1), 1984 (37).
Acknowledgments
Permission from the management of BlueScope Steel for publication of the information contained in this article is gratefully acknowledged. Constructive input provided by the reviewer(s) of this paper is much appreciated.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix I: Diffusion Coefficient of Radioactive Iron in Wüstite Fe1−δ O
From the data of Chen and Peterson [38], the following relationships are obtained,
Regression of the data of Himmel et al. [15] for iron diffusion in Fe0.907O yields,
Note that the original equation given by Himmel et al. could be incorrect, which is, as pointed out previously [1]
Appendix II: Determination of Parameters for Eq. (5)
Extensive studies had been carried out to determine the degree of deviation from stoichiometry for wusttie \({\text{Fe}}_{1 - \delta } {\text{O}}\) [28–35]. At 900 °C, the range of deviation is 1 − δ = 0.89–0.94 [Ref. 36, p. 222].
Himmel et al. [15] conducted experimental studies to determine the relationship between the radioactive trace diffusion coefficient of iron in wüstite and the composition of wüstite at 700 to 983 °C and found that at 900 °C, the value appeared to increase with temperature, although the actual data were rather scattered. Within the range of deviation from stoichiometry (1−δ = 0.89–0.94), the values obtained by Himmel et al. were within the range of 2.35 × 10−8 to 4.45 × 10−8 cm2/s at 900 °C, as shown in Appendix Fig. 8.
Chen and Peterson [38] measured the diffusion coefficients of radioactive iron in wüstite at 800, 1000 and 1200 °C and found that the diffusivity of iron in wüstite increased with δ at 1200 °C, decreased with δ at 800 °C, and insensitive to the variation of δ at 1000 °C. Extrapolating the experimental data to 900 °C using the equations in Appendix I, it was found that the iron diffusivity increased slightly with increased δ, as seen in Appendix Fig. 8, a trend opposite to that claimed by Himmel et al. [15].
Considering the data obtained by Chen and Peterson [38] and those by Himmel et al. [15], it is reasonable to conclude that the diffusivity of iron in wüstite is insensitive to the variation of δ at 900 °C, having a value within the range of 2.5 × 10−8 to 3.0 × 10−8 cm2/s. This conclusion is further supported by the study of Engell [37] who measured the concentration of iron-ion vacancy δ across the wüstite layer electrochemically in the scales formed in oxygen between 750°C and 1000°C and found that the concentration gradient of iron-ion vacancy was constant over the entire wüstite layer. A condition for this to occur, as pointed out by Engell [37], was that the diffusion coefficient of the defects was independent of the oxide concentration. A value of \(D_{\text{Fe}}^{ *} = 2.9 \times 10^{ - 8}\) cm2/s will be used in this study.
There has been no consensus in the determination of the correlation factor for ion diffusion in wüstite [38, 39], although it was proposed [31] that its value was most likely to be within the range of 0.55–0.82, depending on the actual diffusion mechanism (via iron vacancies or interstitial iron ions or both [35, 40]). In conducting theoretical calculation of oxide growth on iron at 1100 °C, Garnaud and Rapp [39] used a correlation factor of 0.5 and obtained good agreement between theoretical and experimental results, whereas in some other studies, \(D_{\text{Fe}}^{ *}\) was treated as the same as \(D_{\text{Fe}}^{\text{s}}\) [1, 3–5, 15]. A value of 0.7 will be used in the current study.
The absence of magnetite precipitates in all the oxidized samples suggests that the oxygen partial pressure was significantly lower than the wüstite/magnetite equilibrium value of 1.83 × 10−15 atm throughout the oxidation period, and therefore the corresponding δ value at the outer surface of the scale would be much smaller than 0.11. Consequently, in the wüstite scales obtained in this study, the value of \(\frac{1}{1 - \delta }\) in Eq. (5) should satisfy the following relationship,
With this small variation range, \(\frac{1}{1 - \delta }\) in Eq. (5) will be approximated as a constant at \(\frac{1}{1 - \delta } \approx 1.07\) or \(1 - \delta \approx 0.935\).
Rights and permissions
About this article
Cite this article
Chen, R.Y., Yuen, W.Y.D. Longer Term Oxidation Kinetics of Low Carbon, Low Silicon Steel in 17H2O–N2 at 900°C. Oxid Met 85, 489–507 (2016). https://doi.org/10.1007/s11085-016-9608-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-016-9608-1