Abstract
During oxidative ladle refining (OLR) of silicon, the metal surface is partly oxidized, resulting in the formation of a condensed silica fume (SiO2). This fugitive emission of silica represents a potential health hazard to the workers in the silicon and ferrosilicon industry. In the current work, industrial measurement campaigns aimed at recording the fume generation during OLR were performed at the Elkem Salten plant in Norway. The measured amounts of silica produced were 2.5–5.1 kg/h, depending on the gas flow rate in the refining process. The rate of silica production correlates with the total flow rate and amount of air in the purge gas, and increases as the flow rate increases. The results of this work suggest that fume generation during OLR primarily results from oxidation of the exposed metal surface, with oxygen transport from the surrounding atmosphere to the metal surface being the limiting factor. Other identified mechanisms of SiO2 formation were splashing of the metal and/or oxidation of SiO gas carried with the refining purge gas.
Similar content being viewed by others
Introduction
An important step in the production of metallurgical grade silicon (MG-Si) is the oxidative ladle treatment of liquid silicon produced in the carbothermic process, with the purpose of purifying the melt from the main impurities, which are aluminum (Al) and calcium (Ca). The treatment is usually performed by purging an air-oxygen mixture through the melt by a nozzle in the bottom of the ladle into which metal is tapped from the electric arc furnace, preferentially oxidizing the impurities into a slag phase [1]. As a result of the contact between liquid silicon and oxygen in the air, silica fume is produced.
While the ladle is under a fume hood, the silica fume formed during refining may be captured. However, when a ladle containing liquid silicon is not kept under a fume hood, or the ladle is transported in the melting hall at the plant, i.e. during casting, the fume is not captured. These fugitive emissions represent a severe indoor environment problem in the silicon and ferrosilicon industry and a potential health hazard for the employees working in the plant [2]. In order to reduce the emissions, a better understanding of the mechanisms and kinetics of the rate of fuming under different conditions, is imperative.
While the oxidation of solid silicon is reasonably well documented due to its importance in the electronic industry, the oxidation behavior of liquid silicon is not widely studied [3]. Wagner [4] studied the phenomenon theoretically in 1958, and presented a well-known theory for the oxidation reaction and its boundary conditions. In 1963, Turkdogan et al. [5] published extensive experimental work on vaporization and oxidation of several liquid metals, and presented a general theory for oxidation of all metals. Wagner and Turkdogan’s theories are widely used and modified in later works, however there does not seem to be a consensus about the boundary conditions and reaction scheme in the Si–O system [6]. The present work is aimed at understanding and determining the rate of oxidation in the industrial processing of silicon. The data will be used, together with existing theories, to develop a model of the oxidation of liquid silicon for industrial prediction of silica fuming rate.
The Mechanisms
When the gas mixture is bubbled through the liquid silicon, there are two possible oxidation reactions occurring in the bubble; the direct oxidation of silicon, where a solid or liquid oxide/slag of silica (SiO2) is produced (passive oxidation, according to Wagner), and the partial oxidation, where the gas silicon monoxide (SiO) is produced (active oxidation, according to Wagner):
By assuming that the activities of silicon in the melt, and silica on the melt-gas bubble interphase are close to unity, the equilibrium constants of reaction (1) and (2) can be used to calculate the equilibrium SiO partial pressure, p SiO(eq), inside the bubbles at a given temperature T, using thermodynamic data from the FactSage thermochemical database [7]:
At a certain critical temperature, T crit, the SiO2 product becomes unstable, and the only oxidized product is SiO(g) due to the reaction
The amounts of SiO and SiO2 formed in and on the bubble, respectively, are expressed in Fig. 1. Above the critical temperature, the only oxidation product is SiO, which leads to no refining and loss of silicon.
A schematic display of the number of moles of SiO and SiO2 formed in the bubble as a function of temperature. In this scenario 1 mol of Si reacts with 0.5 mol of O2 and the pressure is 1 atm. At the critical temperature, here 1,813 °C, the SiO2 production ceases and there is pure loss of silicon to SiO production
In the present case, this critical temperature is only dependent on the amount of oxygen in the purge gas, and increases as the oxygen content in the gas increases. An approximate numerical expression for the critical temperature may be derived from Eq. (3) and the equilibrium constant of reaction (4) [7]:
where x is the initial molar fraction of oxygen in the purge gas, and the temperature is in degrees Celsius. When the bubbles reach the surface of the silicon in the ladle, they burst and the SiO(g) is released into the air above, where it combusts and oxidizes into SiO2(s). Thus, the factor determining the fuming rate by this mechanism is the O2 flow rate. There is a possibility of SiO(g) oxidizing to SiO2(g) inside the bubble prior to the burst at the surface, however due to the instability of SiO2-gas at the temperatures in question, this mechanism has not been considered further.
The actual refining of the silicon takes place with the following exchange reactions
Thus the produced SiO2 at the bubble–liquid metal interface is partly consumed in the refining process, forming an SiO2–Al2O3–CaO slag. The slag is transported to the surface and mixed with the top slag phase due to the buoyancy of the bubble. The refining will lead to a change in composition of both the slag and the silicon, thus a shift in the equilibrium will take place at both the bubble interface and the top metal-slag-atmosphere interface. The initial concentrations of Al and Ca in the metal are typically 1.5 and 0.5 %, respectively, and the end concentrations 0.7 and 0.04 %, respectively [1]. This shift in the equilibrium conditions for the silicon and the slag is however assumed not to affect the equilibrium between the SiO(g) and the SiO2 significantly. This assumption is reasonable because there is little Al/Al2O3 and Ca/CaO compared to SiO2 and SiO(g) in the system, and because the SiO-pressure is only dependent on the temperature in the metal.
Another possible mechanism for fume formation is the surface oxidation of the liquid silicon. Fresh silicon surface is constantly exposed to the ambient atmosphere due to the violent stirring of the melt form gas purging. When oxygen from the surrounding atmosphere meets the surface, reaction (2) will take place and the SiO(g) formed is further combusted in air to become SiO2(s) fume. Following Hinze and Graham [8], this reaction may be written:
In order for the active oxidation (Eq. 2) rather than the passive oxidation (Eq. 1) to occur, there has to be, according to Wagner [4], a significantly lower oxygen partial pressure at the gas-metal interface than in the bulk gas phase, which in the industrial case is ambient air. A complex boundary layer in the gas phase near the surface is described by Ratto et al. [3]. The SiO(g) leaving the interface reacts with the oxygen flowing towards the surface. This heterogeneous boundary layer makes it possible to reach a sufficiently low oxygen partial pressure at the surface of the liquid silicon, even though the bulk oxygen pressure is much higher than the maximum bulk oxygen pressure stated by Wagner (6.1 × 10−3 atm at 1,410 °C). This leads to active oxidation being the preferred oxidation reaction, and Ratto et al. [3] states that the oxygen pressure at the surface will not be dependent on the bulk oxygen pressure. An empirical confirmation of active oxidation is the industrial observation of white fume going off the silicon while it is refined. If the passive oxidation was the sole reaction, the SiO2 produced would be forming a thin layer on the metal surface and it would most likely become a part of the slag phase, and not come off the surface as fume. The rate of silicon surface oxidation with this mechanism will be determined by the rate of oxygen supply to the silicon surface and the size of the exposed melt area.
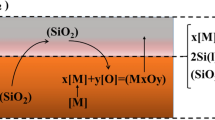
A third possible mechanism for fume formation is splashing of the metal due to drag from the purge bubbles rising with high velocity towards the surface. Visualizing that the dynamic silicon surface looks like a kettle of fast boiling water, and as with water, some very small droplets of silicon may be ejected from the bulk melt. These small silicon droplets are either oxidized and become a part of the fume, or fall to the ground if they are too big and heavy (this is frequently observed). The three possible mechanisms for fume generation in the OLR are schematically visualized in Fig. 2.
In order to quantify the silica fuming during industrial refining of MG-Si under different operational conditions, an industrial measurement campaign was carried out at Elkem’s Salten MG-Si plant in Norway in August 2010. In the present paper, the measurement results from the campaign are presented and discussed in light of existing literature and the presented theories of silica fuming mechanisms.
Experimental
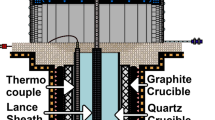
In the measurements it was important to evaluate only the fume formed during the refining of silicon, thus the operationally practiced continuous draining of the furnace was made discontinuous during the measurements. The ladles being refined were placed under the fume hood in order to collect all of the fume in the off-gas channel where the measuring equipment was placed. The measuring equipment was a LaserDust MP, a commercial apparatus from NEO Monitors (Lørenskog, Norway) [9]. The LaserDust measures the transmission of a laser beam, through for example an off gas duct/channel, and the reduction in the signal will correspond to the amount of fume in the measuring path. This change in signal is dependent on the properties of the particles, primarily the particle size distribution. The LaserDust used was not calibrated particularly for silica fume, however the measurements are evaluated to have a total measuring error of 30 %, which is in the same order of magnitude as the typical standard deviations in the measurements. The measuring path was 1.2 m (the width of the off gas channel). The LaserDust was programmed with the pressure and temperature, which on the day of our measurements was 1,013 mbar and approximately 50 °C (in the off gas channel). The apparatus had a relative error of 2 % in the measurements, and measured the fume amount in the off-gas channel every 5 s [10].
In order to relate the rate of the fume formation to the stirring occurring in the ladle, the rate and composition of the purge gas were varied. A summary of the chosen conditions for the six measurements are shown in Table 1. One of the ladles was refined with a semi-lid in order to see whether this would affect the fume amount (measurements 4–6 in Table 1). The semi lid covered the perimeter of the ladle, and had a hole with a diameter of ~60 cm in the center for practical operational purposes.
The output unit from the LaserDust MP, mg/m3, was converted to kg/h by implementing the measured temperature (~50 °C) and the measured volume flow in the pipes (50.000 Nm3/h). The output in mg/m3 was multiplied with the calculated factor of 11.2 m3/s in order to obtain the mass per time unit.
In addition to measurement of the amount of silica, videotapes of the refining process were recorded. At the time of the measurements, the temperature in the melt was in the range of 1,450–1,500 °C. Gas temperature measured at approximately 0.5 m above the silicon was in the range of 370–500 °C. Figure 3 shows how a ladle top (silicon surface) typically looks like during refining, and the location of the LaserDust MP at the off gas channel, approximately 20 m from the ladle top. There is a layer of slag and solidified silicon along the perimeter of the melt, and the exposed area of liquid silicon was typically in the range 0.3–0.5 m2, when taking into account that the surface is convex from the bubble stirring.
Silica fume was collected in the off-gas channel during tapping and refining with a “cold finger” (a hollow copper tube cooled with water). The fumes were examined using a scanning electron microscope (SEM) and compared to samples of silica taken from the furnace stack. The SEM used was a Zeiss Ultra 55 Limited Edition field emission microscope. The samples were held in place by a carbon tape in order to avoid charging, and the images were recorded at an acceleration voltage of 2–5 kV, with magnifications 2k–50k. From the SEM images, the particle size distribution and mean diameter of the particles were estimated. The SEM apparatus was also used to perform element analysis of the samples with energy dispersive spectroscopy (EDS). The EDS-spectra were recorded at 20 kV.
The fume was also examined with a transmission electron microscope (TEM) in order to confirm that the silica was amorphous and to see if the particles were hollow. The TEM used was a JEOL JEM2010. The samples were dispersed on a Holey Carbon Film 300 mesh Cu from Agar Scientific, and the images were recorded at 200 kV.
The specific surface area of the fume samples were measured with BET (an adsorption theory and method developed by Brunauer, Emmett and Teller) in order to find out whether the particles were porous or not, and to compare the specific surface area to that of the silica samples taken from the furnace stack (this was not possible for the sample collected during refining, due to too small sample size). The samples were degassed over night at 250 °C under vacuum in a VacPrep 061 from Micromeretics, and analyzed with liquid nitrogen in a Tristar™ 3000 from Micromeretics.
Results and Discussion
The calculated average amounts of SiO2 measured for the different experimental conditions are shown in Table 2. The maximum and minimum amounts were calculated with a combined error function (standard deviation and the total measurement error of 30 % combined). The “macro” area of exposed silicon at the top surface was estimated from the videotapes of the refining, and used to calculate the flux of silica (moles/s m2). Due to the vigorous stirring, this estimated area is underestimated compared to a true metal-gas surface area, but it gives an indication of the silicon surface area not covered by slag.
Plots of the SiO2 produced per unit time as a function of total gas flow rate, air and oxygen flow rates are shown in Fig. 4.
Plots of the measured amount of silica fume (kg/h) versus the amount of gas total (left), air (middle) and oxygen (right) in m3/h. The regression lines for all measurements (solid lines), and for ladles with lid (dotted lines) and without lid (dashed lines), are shown. The squared correlations (R 2) for all measurements (solid lines) are given in the upper right corner of each graph, and the shape of the points indicates whether a lid (triangles) or no lid (diamonds) was present
The amount of fume formed during refining correlates best with the amount of air in the purge gas, and also adequately with the total amount of purge gas. The difference between the fume amount with (dotted lines, triangles) and without a lid (dashed lines, diamonds) is not significant, thus the amount of oxygen available to the surface in not limited by the lid in the current conditions. The lid only serves to concentrate the fume in order to capture it better with the ventilation off-gas channel. The reason for the increased amount of fume with increasing amount of purge gas is likely due to more stirring and thus a larger area of the surface will be exposed to air, available for oxidation. This effect is observed both with and without a lid, the oxygen availability is thus assumed to be similar in both cases.
The results also indicate that the fuming rate is not dependent on the oxygen content in the purge gas (neither the input amount nor the total amount, which is not shown here). According to Eq. (3), the calculated equilibrium SiO(g) partial pressure in the bubbles corresponds to 0.02–0.11 kg of SiO2 per hour, using the experimental gas rates and compositions. Compared to the measured total of 2.5–5.1 kg of SiO2 per hour, it was concluded that oxidation of SiO(g) from the purge gas bubbles is not the dominating mechanism for fume formation during silicon refining.
Gulbransen and Jansson [12] measured a flux of 0.0538 mol/s m2 at 1,300 °C, Ptot = 1.3 × 10−4 atm and with an oxygen flow of 7.0 × 1018 molecules per second (corresponds to ~11.4 L/s, or a flow rate of 21 m/s). Although this was measured on solid silicon, the flux is in the same order of magnitude as the estimated fluxes in this work.
Images from SEM and TEM analysis of silica fume are shown in Fig. 5. The silica from the refining and tapping looked exactly the same as condensed silica fume; the particles are perfect spheres with a size distribution of 0.001–3 μm, with 70–90 % of the particles being <0.05 μm in size. This is somewhat smaller than reported in literature [13]. The particle size distributions for the three different samples are shown in Fig. 6. The TEM (right part of Fig. 5) showed that the particles were amorphous, as expected, and also that they had no pores or were hollow. In the figure it is seen that there is no ordered crystal structure of the atoms in the particles, and the smallest particles are ~10 nm in diameter.
The particles in the collected samples and the silica samples taken from the furnace stack had very similar particle size distribution. However, the samples collected above the ladle during refining and in the off-gas channel during tapping, contained some few particles with sizes of 20–70 μm. These particles were also spherical, as shown in Fig. 7, however, they contained some iron and aluminum, in addition to silicon, oxygen and carbon. This type of particles was not found in the silica samples taken from the furnace stack. As seen in the upper right inset in Fig. 7, the surface of these large particles had a rough, layered texture, possibly due to oxidation of the surface of a silicon droplet. It is suggested that these large particles are created from splashing of the metal due to bubble drag (mechanism b in Fig. 2). The presence of Fe and Al supports this, as these elements will predominantly follow the metal and not the fume, in addition to the fact that these large particles were not found in the silica samples taken from the furnace stack.
The specific surface area of the samples measured by BET were 17.61 ± 0.05 m2/g for the silica samples taken from the furnace stack, and 14.90 ± 0.04 m2/g for silica fume collected during tapping. These areas are in the same order of magnitude, and the fairly low values signify that the particles are not porous. The similar specific surface area, and similar particle size distribution indicates that the particles in the fume samples from the refining and tapping are generated from the same mechanism as the silica from the furnace stack; SiO(g) oxidation. It is well known that the silica fume from the furnace stack is produced by the combustion of SiO rising up through the furnace into the off-gas channel together with the other gases from the silicon production (CO and small amounts of e.g. SO2 and NO x ). This supports our theory of the active oxidation on the liquid silicon surface (mechanism c in Fig. 2).
In a Computational Fluid Dynamics (CFD) study, Olsen et al. [14] have modeled the ladle refining and performed a sensitivity study in order to map the factors affecting the fuming rate significantly. They tested temperature, emissivity of the silicon surface, different lid designs and exposed metal surface area. In the study they found that metal temperature is not an important factor, as the temperature effect on diffusion and/or convection will counteract the increased reaction rate. The CFD modeling concluded that exposed surface area was the most significant factor in the fuming rate, and that oxygen availability is the limiting factor in the oxidation [14]. These results support the findings of the present study, as the results suggest surface oxidation to be the dominant oxidation mechanism in OLR.
Conclusions
The amount of silica fume produced during industrial refining of MG-Si has been measured. In order to correlate the fuming rate to stirring in the molten silicon, the gas purging rate and composition were varied. The amount of fume was recorded with a LaserDust MP, which was installed in the off-gas channel. The measurements had an assumed total measurement error of 30 %. Six different measurements were carried out, with recorded fume amounts of 2.5–5.1 kg of SiO2 per hour. The results indicate that the fuming rate is correlated with the amount of purge gas.
Three mechanisms for the fume formation were proposed and evaluated (see Fig. 2): Fume produced from oxidation of SiO-gas from the refining bubbles was found to be less significant compared to the recorded amount of fume. Splashing of metal droplets due to bubble drag is a contributing mechanism, however, the dominating mechanism is believed to be the surface oxidation of the molten silicon. Increased amount of purge gas leads to more vigorous stirring and larger exposed surface area available for oxidation with oxygen from the air. The results indicate that refining with and without a semi-lid on the ladle do not affect the amount of fume formed, and a lid will thus not limit the oxygen supply to the surface significantly. Even though the bulk oxygen pressure in air is too high for active oxidation to occur (the Wagner theory), active oxidation would still be possible if a complex heterogeneous boundary layer is present, where most of the oxygen is consumed by oxidation of SiO(g) to SiO2, prior to the liquid silicon surface.
The primary particles in the fume had a size distribution of 0.01–3 μm, with 70–90 % of the particles being <0.05 μm in diameter. The particles are amorphous and non-porous. The similarity between the particles in the fume samples collected during tapping and refining, and the silica samples collected from the furnace stack, suggest that they are formed from the same mechanism; oxidation of SiO(g). This finding supports the theory of the surface oxidation being the dominant mechanism in the fuming during OLR.
The conclusion that the surface oxidation is the dominating oxidation mechanism is supported by a CFD study performed by Olsen et al. in [14], where it was concluded that the exposed silicon surface area is the most important factor in the fuming rate, and that the oxygen availability is the limiting factor to the oxidation.
The results in this paper have given a better understanding of the mechanism from which the fugitive emissions of silica during OLR are generated. The amounts of silica emitted have been quantified. Further work will be small scale experiments in order to better understand the fundamental mechanism of the oxidation, and in order to better characterize the boundary layer and the boundary conditions for the active oxidation.
References
A. Schei, J. K. Tuset, and H. Tveit, Production of High Silicon Alloys (TAPIR forlag, Trondheim) (1998).
H. L. Johnsen, Lung Function, Respiratory Symptoms, and Occupational Exposure. Ph.D. Thesis. University of Oslo, Oslo (2009).
M. Ratto, E. Ricci, E. Arato and P. Costa, Oxidation of metals with highly reactive vapors: Extension of Wagner theory. Metallurgical and Materials Transactions B 32, 903 (2001).
C. Wagner, Passivity during the oxidation of silicon at elevated temperatures. Journal of Applied Physics 29, 1295 (1958).
E. T. Turkdogan, P. Grieveson and L. S. Darken, Enhancement of diffusion-limited rates of vaporization of metals. Journal of Physical Chemistry 67, 1647 (1963).
K. Hildal, (2002). Steam explosions during granulation of Si-rich alloys: Effect of Ca- and Al-additions. Ph.D. Thesis. Norwegian University of Science and Technology (NTNU), Trondheim.
K. Tang, Thermodynamic analysis of oxidative ladle refining of silicon melt including models of thermophysical properties of the silicon melt and SiO 2 –Al 2 O 3 –CaO slag, (SINTEF, Trondheim, 2007).
J. W. Hinze and H. C. Graham, The active oxidation of Si and SiC in the viscous gas-flow regime. Journal of the Electrochemical Society 123, 1066 (1976).
NEO Monitors [Online]. Lørenskog: NEO Monitors. Available: www.neomonitors.com/products/dust-cross-stack/laserdust/ [Accessed 20.02.2012].
P. Kaspersen, Personal Communication, (Norsk Elektro Optikk AS (NEO), Lørenskog) (2010).
A. Ashrafian, S. T. Johansen, S. Gaal, B. Andresen and O. S. Klevan, A reactor model for ladle refining of silicon melt. 6th international Conference on CFD in Oil and Gas, Metallurgical and Process Industries, SINTEF/NTNU, Trondheim, Norway, 10–12. June, 2008.
E. A. Gulbransen and S. A. Jansson, The high-temperature oxidation, reduction, and volatilization reactions of silicon and silicon carbide. Oxidation of metals 4, 181 (1972).
E. Dingsøyr, M. Dåstøl and W. C. Wedberg, Particle size and particle size distribution of Elkem Microsilica and its relevance to technical application. The 5th European Symposium Particle Characterization, Nurnberg, Germany, 24–26. March, 1992.
J. E. Olsen, M. Næss and G. Tranell, CFD modeling of silica fume formation during refining of silicon metal. 8th International Conference on CFD in Oil and Gas, Metallurgical and Process Industries, SINTEF/NTNU, Trondheim, Norway, 21–23. June 2011.
Acknowledgments
The financing of this work was provided by the Norwegian Research Council and FFF (Ferro Alloys Industries Research Association) through the FUME project (Fugitive emissions of Materials and Energy). We would like to express our appreciation to SINTEF for their contribution to this work, and Elkem for allowing us to carry out the measurements at their plant in Salten. Also we would like to thank Norsk Elektro Optikk AS (NEO) for using their equipment, and for assisting in the technical part of the measurements.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Næss, M.K., Tranell, G., Olsen, J.E. et al. Mechanisms and Kinetics of Liquid Silicon Oxidation During Industrial Refining. Oxid Met 78, 239–251 (2012). https://doi.org/10.1007/s11085-012-9303-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-012-9303-9