Abstract
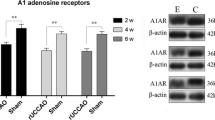
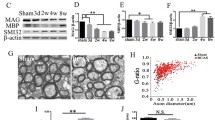
We sought to investigate the role of the adenosine A1 receptors (A1ARs) in white matter lesions under chronic cerebral hypoperfusion (CCH) and explore the potential repair mechanisms by activation of the receptors. A right unilateral common carotid artery occlusion (rUCCAO) method was used to construct a CCH model. 2-chloro-N6-cyclopentyladenosine (CCPA), a specific agonist of A1ARs, was used to explore the biological mechanisms of repair in white matter lesions under CCH. The expression of mammalian target of rapamycin (mTOR), phosphorylation of mTOR (P-mTOR), myelin basic protein (MBP, a marker of white matter myelination) were detected by Western-blot. Pro-inflammatory cytokine tumor necrosis factor-α (TNF-α) and anti-inflammatory cytokine interleukin-10 (IL-10) levels were determined by ELISA. Compared with the control groups on week 2, 4 and 6, in CCPA-treated groups, the ratio of P-mTOR/mTOR, expression of MBP and IL-10 increased markedly, while the expression of TNF-α reduced at week 6. In conclusion, A1ARs appears to reduce inflammation in white matter via the mTOR signaling pathway in the rUCCAO mice. Therefore, A1ARs may serve as a therapeutic target during the repair of white matter lesions under CCH.




Similar content being viewed by others
References
Garde E, Mortensen EL, Krabbe K, Rostrup E, Larsson HB (2000) Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet 356:628–634
Wang J, Zhang HY, Tang XC (2010) Huperzine a improves chronic inflammation and cognitive decline in rats with cerebral hypoperfusion. J Neurosci Res 88(4):807–815
Thorn JA JS (1996) Adenosine transporters. Gen Pharmacol 27:613–620
Cunha RA (2005) Neuroprotection by adenosine in the brain: from A1 receptor activation to A2A receptor blockade. Purinergic signal 1:111–134
Johnston JB, Silva C, Gonzalez G et al (2001) Diminished adenosine A1 receptor expression on macrophages in brain and blood of patients with multiple sclerosis. Ann Neurol 49:650–658
Tsutsui S, Schnermann J, Noorbakhsh F et al (2004) A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J Neurosci 24:1521–1529
Cheng P, Ren Y, Bai S et al (2015) Chronic cerebral ischemia induces downregulation of A1 adenosine receptors during white matter damage in adult mice. Cell Mol Neurobiol 35(8):1149–1156
Tyler WA, Gangoli N, Gokina P et al (2009) Activation of the mammalian target of rapamycin (mTOR) is essential for oligodendrocyte differentiation. J Neurosci 29:6367–6378
Yoshizaki K, Adachi K, Kataoka S, Watanabe A, Tabira T, Takahashi K, Wakita H (2008) Chronic cerebral hypoperfusion induced by right unilateral common carotid artery occlusion causes delayed white matter lesions and cognitive impairment in adult mice. Exp Neurol 210(2):585–591
Zhao T, Xi L, Chelliah J, Levasseur JE, Kukreja RC (2000) Inducible nitric oxide synthase mediates delayedmyocardial protection induced by activation of adenosine A1 receptors evidence from gene-knockout mice. Circulation 102:902–907
Chong ZZ, Shang YC, Zhang L, Wang S, Maiese K (2010) Mammalian target of rapamycin: hitting the bull’s-eye for neurological disorders. Oxid Med Cell Longev 3:374–391
Floyd S, Favre C, Lasorsa FM et al (2007) The insulin-like growth factor-I–mTOR signaling pathway induces the mitochondrial pyrimidine nucleotide carrier to promote cell growth. Mol Biol Cell 18:3545–3555
Good DW, George T, Watts BA (2008) Nerve growth factor inhibits Na+/H+ exchange and absorption through parallel phosphatidylinositol 3-kinase-mTOR and ERK pathways in thick ascending limb. J Biol Chem 283:26602–26611
Han S, Witt RM, Santos TM et al (2008) Pam (protein associated with Myc) functions as an E3 ubiquitin ligase and regulates TSC/mTOR signaling. Cell Signal 20:1084–1091
Recchia AG, Musti AM, Lanzino M et al (2009) A cross-talk between the androgen receptor and the epidermal growth factor receptor leads to p38MAPK-dependent activation of mTOR and cyclinD1 expression in prostate and lung cancer cells. Int J Biochem Cell Biol 41:603–614
Choi KC, Kim SH, Ha JY, Kim ST, Son JH (2010) A novel mTOR activating protein protects dopamine neurons against oxidative stress by repressing autophagy related cell death. J Neurochem 112:366–376
Shang YC, Chong ZZ, Wang S, Maiese K (2012) Prevention of β-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging 4:187
Chano T, Okabe H, Hulette CM (2007) RB1CC1 insufficiency causes neuronal atrophy through mTOR signaling alteration and involved in the pathology of Alzheimer’s diseases. Brain Res 1168:97–105
Dwyer JM, Lepack AE, Duman RS (2012) mTOR activation is required for the antidepressant effects of mGluR2/3 blockade. Int J Neuropsychopharmacol 15:429–434
Li N, Liu RJ, Dwyer JM et al (2011) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761
Hoeffer CA, Klann E (2010) mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 33:67–75
Jaworski J, Sheng M (2006) The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol 34:205–219
Puighermanal E, Marsicano G, Busquets-Garcia A et al (2009) Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci 12:1152–1158
Swiech, L., Perycz, M., Malik, A. & Jaworski, J (2008) Role of mTOR in physiology and pathology of the nervous system. Biochim Biophys Acta (BBA) Proteins Proteomics 1784:116–132
Blundell J, Kouser M, Powell CM (2008) Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol Learn Mem 90:28–35
Huang Z, Liu J, Cheung P-Y, Chen C (2009) Long-term cognitive impairment and myelination deficiency in a rat model of perinatal hypoxic-ischemic brain injury. Brain Res 1301:100–109
Wang DS, Bennett DA, Mufson EJ et al (2004) Contribution of changes in ubiquitin and myelin basic protein to age-related cognitive decline. Neurosci Res 48:93–100
Yang J, Jiang Z, Fitzgerald DC et al (2009) Adult neural stem cells expressing IL-10 confer potent immunomodulation and remyelination in experimental autoimmune encephalitis. J Clin Invest 119:3678
Rodts-Palenik S, Wyatt-Ashmead J, Pang Y et al (2004) Maternal infection-induced white matter injury is reduced by treatment with interleukin-10. Am J Obstet Gynecol 191:1387–1392
Boyd ZS, Kriatchko A, Yang J et al (2003) Interleukin-10 receptor signaling through STAT-3 regulates the apoptosis of retinal ganglion cells in response to stress. Invest Ophthalmol Vis Sci 44:5206–5211
Molina-Holgado F, Grencis R, Rothwell NJ (2001) Actions of exogenous and endogenous IL-10 on glial responses to bacterial LPS/cytokines. Glia 33:97–106
Strle K, Zhou JH, Shen WH et al (2001) lnterleukin-10 in the brain. Crit Rev Immunol 21(5):427–449
Hasko G, Szabó C, Németh ZH et al (1996) Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol 157:4634–4640
Weichhart T, Costantino G, Poglitsch M et al (2008) The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 29:565–577
Rodriguez-Yanez M, Castillo J (2008) Role of inflammatory markers in brain ischemia. Curr Opin Neurol 21:353–357. doi:10.1097/WCO.0b013e3282ffafbf
Acknowledgments
This work was supported by research Grants from the National Natural Science Foundation of China (Grant # 81171113, Grant # 81571129). We thank the laboratory support staff for their help in this study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Pengfei Cheng and Xuzheng Zuo have contributed equally to the work.
Rights and permissions
About this article
Cite this article
Cheng, P., Zuo, X., Ren, Y. et al. Adenosine A1-Receptors Modulate mTOR Signaling to Regulate White Matter Inflammatory Lesions Induced by Chronic Cerebral Hypoperfusion. Neurochem Res 41, 3272–3277 (2016). https://doi.org/10.1007/s11064-016-2056-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2056-0