Abstract
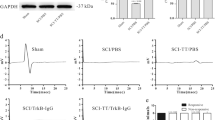
Spinal cord injury (SCI), a severe health problem in worldwide, was commonly associated with functional disability and reduced quality of life. As the expression of brain-derived neurotrophic factor (BDNF) was substantial event in injured spinal cord, we hypothesized whether BDNF-overexpression could be in favor of the recovery of both sensory function and hindlimb function after SCI. By using BDNF-overexpression transgene mice [CMV-BDNF 26 (CB26) mice] we assessed the role of BDNF on the recovery of neurological behavior in spinal cord transection (SCT) model. BMS score and tail-flick test was performed to evaluate locomotor function and sensory function, respectively. Immunohistochemistry was employed to detect the location and the expression of BDNF, NeuN, 5-HT, GAP-43, GFAP as well as CGRP, and the level of p-AKT and AKT were examined through western blot analysis. BDNF overexpressing resulted in significant locomotor functional recovery from 21 to 28 days after SCT, compared with wild type (WT)+SCT group. Meanwhile, the NeuN, 5-HT and GAP-43 positive cells were markedly increased in ventral horn in BDNF overexpression animals, compared with WT mice with SCT. Moreover, the crucial molecular signal, p-AKT/AKT has been largely up-regulated, which is consistent with the improvement of locomotor function. However, in this study, thermal hyperpathia encountered in sham (CB26) group and WT+SCT mice and further aggravated in CB26 mice after SCT. Also, following SCT, the significant augment of positive-GFAP astrocytes and CGRP fibers were found in WT+SCT mice, and further increase was seen in BDNF over-expression transgene mice. BDNF-overexpression may not only facilitate the recovery of locomotor function via AKT pathway, but also contributed simultaneously to thermal hyperalgesia after SCT.







Similar content being viewed by others
References
Ellaway PH, Vasquez N, Craggs M (2014) Induction of central nervous system plasticity by repetitive transcranial magnetic stimulation to promote sensorimotor recovery in incomplete spinal cord injury. Front Integr Neurosci 8:42. doi:10.3389/fnint.2014.00042
Rosner J, Avalos P, Acosta F, Liu J, Drazin D (2012) The potential for cellular therapy combined with growth factors in spinal cord injury. Stem Cells Int 2012:826754. doi:10.1155/2012/826754
Sekhon LH, Fehlings MG (2001) Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 26:S2–12
Geisler FH, Dorsey FC, Coleman WP (1991) Recovery of motor function after spinal-cord injury––a randomized, placebo-controlled trial with GM-1 ganglioside. N Engl J Med 324:1829–1838. doi:10.1056/nejm199106273242601
Liu R, Zhao W, Zhao Q, Liu SJ, Liu J, He M, Xu Y, Wang W, Liu W, Xia QJ, Li CY, Wang TH (2014) Endoplasmic reticulum protein 29 protects cortical neurons from apoptosis and promoting corticospinal tract regeneration to improve neural behavior via caspase and Erk signal in rats with spinal cord transection. Mol Neurobiol 50:1035–1048. doi:10.1007/s12035-014-8681-1
Gransee H, Zhan WZ, Sieck GC, Mantilla C (2014) Localized delivery of BDNF-expressing mesenchymal stem cells enhances functional recovery following cervical spinal cord injury. J Neurotrauma. doi:10.1089/neu.2014.3464
Sieck GC, Mantilla CB (2009) Role of neurotrophins in recovery of phrenic motor function following spinal cord injury. Respir Physiol Neurobiol 169:218–225. doi:10.1016/j.resp.2009.08.008
Lu B, Figurov A (1997) Role of neurotrophins in synapse development and plasticity. Rev Neurosci 8:1–12
Wardle RA, Poo MM (2003) Brain-derived neurotrophic factor modulation of GABAergic synapses by postsynaptic regulation of chloride transport. J Neurosci 23:8722–8732
Ying Z, Roy RR, Zhong H, Zdunowski S, Edgerton VR, Gomez-Pinilla F (2008) BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience 155:1070–1078. doi:10.1016/j.neuroscience.2008.06.057
Xing RX, Zhu LL, Yang M, Liu F (2015) BDNF administration in skeletal muscle is effective to improve locomotor function in SCT rats. Ibrain 2:1–8
Liu J, Liu F, Zhang ZB, Xiong LL, Luo CZ (2015) HSV-BDNF releasing in local motor neuron increases locomotor function in SCT rats. Ibrain 2:1–7
Sun R, Yan J, Willis WD (2007) Activation of protein kinase B/Akt in the periphery contributes to pain behavior induced by capsaicin in rats. Neuroscience 144:286–294. doi:10.1016/j.neuroscience.2006.08.084
Duric V, McCarson KE (2006) Effects of analgesic or antidepressant drugs on pain- or stress-evoked hippocampal and spinal neurokinin-1 receptor and brain-derived neurotrophic factor gene expression in the rat. J Pharmacol Exp Ther 319:1235–1243. doi:10.1124/jpet.106.109470
Gao L, Li LH, Xing RX, Ou S, Liu GD, Wang YP, Zhang H, Gao GD, Wang TH (2012) Gastrocnemius-derived BDNF promotes motor function recovery in spinal cord transected rats. Growth Factors 30:167–175. doi:10.3109/08977194.2012.678842
Feng GY, Xia QJ, Wang TH (2015) PCR technology, how do you get it? Idiscovery 2:32–41
Chang YC, Rapoport SI, Rao JS (2009) Chronic administration of mood stabilizers upregulates BDNF and bcl-2 expression levels in rat frontal cortex. Neurochem Res 34:536–541. doi:10.1007/s11064-008-9817-3
Murakami T, Kanchiku T, Suzuki H, Imajo Y, Yoshida Y, Nomura H, Cui D, Ishikawa T, Ikeda E, Taguchi T (2013) Anti-interleukin-6 receptor antibody reduces neuropathic pain following spinal cord injury in mice. Exp Ther Med 6:1194–1198. doi:10.3892/etm.2013.1296
Dong XJ, Liu J, Sun ZW, Zhou LH, Wang TH (2015) Technology of protein quantification, how do we know? Idiscovery 2(2):31–38
Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S (2006) Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci USA 103:13208–13213. doi:10.1073/pnas.0605180103
Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S (1999) BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98:739–755
Xie Y, Hayden MR, Xu B (2010) BDNF overexpression in the forebrain rescues Huntington’s disease phenotypes in YAC128 mice. J Neurosci 30:14708–14718. doi:10.1523/jneurosci.1637-10.2010
Ge F, Yao J, Fu X, Guo Z, Yan J, Zhang B, Zhang H, Tomozawa H, Miyazaki J, Sawashita J, Mori M, Higuchi K (2007) Amyloidosis in transgenic mice expressing murine amyloidogenic apolipoprotein A-II (Apoa2c). Lab Invest 87:633–643. doi:10.1038/labinvest.3700559
Takasawa A, Kato I, Takasawa K, Ishii Y, Yoshida T, Shehata MH, Kawaguchi H, Mohafez OM, Sasahara M, Hiraga K (2008) Mutation-, aging-, and gene dosage-dependent accumulation of neuroserpin (G392E) in endoplasmic reticula and lysosomes of neurons in transgenic mice. J Biol Chem 283:35606–35613. doi:10.1074/jbc.M804125200
Song XY, Li F, Zhang FH, Zhong JH, Zhou XF (2008) Peripherally-derived BDNF promotes regeneration of ascending sensory neurons after spinal cord injury. PLoS One 3:e1707. doi:10.1371/journal.pone.0001707
Ziemlinska E, Kugler S, Schachner M, Wewior I, Czarkowska-Bauch J, Skup M (2014) Overexpression of BDNF increases excitability of the lumbar spinal network and leads to robust early locomotor recovery in completely spinalized rats. PLoS One 9:e88833. doi:10.1371/journal.pone.0088833
Samantaray S, Das A, Matzelle DC, Yu SP, Wei L, Varma A, Ray SK, Banik NL (2016) Administration of low dose-estrogen attenuates persistent inflammation, promotes angiogenesis and improves locomotor function following chronic spinal cord injury in rats. J Neurochem. doi:10.1111/jnc.13610
Colon JM, Torrado AI, Cajigas A, Santiago JM, Salgado IK, Arroyo Y, Miranda JD (2016) Tamoxifen administration immediately or 24 h after spinal cord injury improves locomotor recovery and reduces secondary damage in female rats. J Neurotrauma. doi:10.1089/neu.2015.4111
Alizadeh A, Karimi-Abdolrezaee S (2016) Microenvironmental regulation of oligodendrocyte replacement and remyelination in spinal cord injury. J Physiol. doi:10.1113/jp270895
Chen MH, Liu YH, Xu H, Xu DW, Wang CN, Wang Y, Duan CW, Zhou Y, Kan P, Shen AG, Wang YH (2015) Lentiviral vector-mediated p27 expression facilitates recovery after spinal cord injury. Mol Neurobiol. doi:10.1007/s12035-015-9498-2
Han Q, Sun W, Lin H, Zhao W, Gao Y, Zhao Y, Chen B, Xiao Z, Hu W, Li Y, Yang B, Dai J (2009) Linear ordered collagen scaffolds loaded with collagen-binding brain-derived neurotrophic factor improve the recovery of spinal cord injury in rats. Tissue Eng Part A 15:2927–2935. doi:10.1089/ten.TEA.2008.0506
Lee YS, Lin CY, Caiozzo VJ, Robertson RT, Yu J, Lin VW (2007) Repair of spinal cord transection and its effects on muscle mass and myosin heavy chain isoform phenotype. J Appl Physiol (1985) 103:1808–1814. doi:10.1152/japplphysiol.00588.2007
Tom VJ, Sandrow-Feinberg HR, Miller K, Domitrovich C, Bouyer J, Zhukareva V, Klaw MC, Lemay MA, Houle JD (2013) Exogenous BDNF enhances the integration of chronically injured axons that regenerate through a peripheral nerve grafted into a chondroitinase-treated spinal cord injury site. Exp Neurol 239:91–100. doi:10.1016/j.expneurol.2012.09.011
Teshigawara K, Kuboyama T, Shigyo M, Nagata A, Sugimoto K, Matsuya Y, Tohda C (2013) A novel compound, denosomin, ameliorates spinal cord injury via axonal growth associated with astrocyte-secreted vimentin. Br J Pharmacol 168:903–919. doi:10.1111/j.1476-5381.2012.02211.x
Jacobs BL, Martin-Cora FJ, Fornal CA (2002) Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev 40:45–52
Ghosh M, Pearse DD (2014) The role of the serotonergic system in locomotor recovery after spinal cord injury. Front Neural Circuits 8:151. doi:10.3389/fncir.2014.00151
Leech KA, Kinnaird CR, Hornby TG (2014) Effects of serotonergic medications on locomotor performance in humans with incomplete spinal cord injury. J Neurotrauma 31:1334–1342. doi:10.1089/neu.2013.3206
Duric V, McCarson KE (2007) Neurokinin-1 (NK-1) receptor and brain-derived neurotrophic factor (BDNF) gene expression is differentially modulated in the rat spinal dorsal horn and hippocampus during inflammatory pain. Mol Pain 3:32. doi:10.1186/1744-8069-3-32
Blanquet PR, Mariani J, Derer P (2003) A calcium/calmodulin kinase pathway connects brain-derived neurotrophic factor to the cyclic AMP-responsive transcription factor in the rat hippocampus. Neuroscience 118:477–490
Bulsara KR, Iskandar BJ, Villavicencio AT, Skene JH (2002) A new millenium for spinal cord regeneration: growth-associated genes. Spine (Phila Pa 1976) 27:1946–1949
Watson JL, Hala TJ, Putatunda R, Sannie D, Lepore AC (2014) Persistent at-level thermal hyperalgesia and tactile allodynia accompany chronic neuronal and astrocyte activation in superficial dorsal horn following mouse cervical contusion spinal cord injury. PLoS One 9:e109099. doi:10.1371/journal.pone.0109099
Gruber HE, Ingram JA, Hoelscher G, Zinchenko N, Norton HJ, Hanley EN Jr (2008) Brain-derived neurotrophic factor and its receptor in the human and the sand rat intervertebral disc. Arthritis Res Ther 10:R82. doi:10.1186/ar2456
Obata K, Noguchi K (2006) BDNF in sensory neurons and chronic pain. Neurosci Res 55:1–10. doi:10.1016/j.neures.2006.01.005
Naseri K, Saghaei E, Abbaszadeh F, Afhami M, Haeri A, Rahimi F, Jorjani M (2013) Role of microglia and astrocyte in central pain syndrome following electrolytic lesion at the spinothalamic tract in rats. J Mol Neurosci 49:470–479. doi:10.1007/s12031-012-9840-3
Hoschouer EL, Yin FQ, Jakeman LB (2009) L1 cell adhesion molecule is essential for the maintenance of hyperalgesia after spinal cord injury. Exp Neurol 216:22–34. doi:10.1016/j.expneurol.2008.10.025
Vanegas H, Schaible HG (2001) Prostaglandins and cyclooxygenases (correction of cycloxygenases) in the spinal cord. Prog Neurobiol 64:327–363
McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA (2007) Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 1773:1263–1284. doi:10.1016/j.bbamcr.2006.10.001
Tan Y, Uchida K, Nakajima H, Guerrero AR, Watanabe S, Hirai T, Takeura N, Liu SY, Johnson WE, Baba H (2013) Blockade of interleukin 6 signaling improves the survival rate of transplanted bone marrow stromal cells and increases locomotor function in mice with spinal cord injury. J Neuropathol Exp Neurol 72:980–993. doi:10.1097/NEN.0b013e3182a79de9
Sun Z, Hu L, Wen Y, Chen K, Sun Z, Yue H, Zhang C (2013) Adenosine triphosphate promotes locomotor recovery after spinal cord injury by activating mammalian target of rapamycin pathway in rats. Neural Regen Res 8:101–110. doi:10.3969/j.issn.1673-5374.2013.02.001
Hu LY, Sun ZG, Wen YM, Cheng GZ, Wang SL, Zhao HB, Zhang XR (2010) ATP-mediated protein kinase B Akt/mammalian target of rapamycin mTOR/p70 ribosomal S6 protein p70S6 kinase signaling pathway activation promotes improvement of locomotor function after spinal cord injury in rats. Neuroscience 169:1046–1062. doi:10.1016/j.neuroscience.2010.05.046
Li L, Guo JD, Wang HD, Shi YM, Yuan YL, Hou SX (2015) Prohibitin 1 gene delivery promotes functional recovery in rats with spinal cord injury. Neuroscience 286:27–36. doi:10.1016/j.neuroscience.2014.11.037
Penumatsa K, Abualkhair S, Wei L, Warburton R, Preston I, Hill NS, Watts SW, Fanburg BL, Toksoz D (2014) Tissue transglutaminase promotes serotonin-induced AKT signaling and mitogenesis in pulmonary vascular smooth muscle cells. Cell Signal 26:2818–2825. doi:10.1016/j.cellsig.2014.09.002
Saunders C, Siuta M, Robertson SD, Davis AR, Sauer J, Matthies HJ, Gresch PJ, Airey DC, Lindsley CW, Schetz JA, Niswender KD, Veenstra-Vanderweele JM, Galli A (2014) Neuronal ablation of p-Akt at Ser473 leads to altered 5-HT1A/2A receptor function. Neurochem Int 73:113–121. doi:10.1016/j.neuint.2013.09.015
Zhao S, Fu J, Liu X, Wang T, Zhang J, Zhao Y (2012) Activation of Akt/GSK-3beta/beta-catenin signaling pathway is involved in survival of neurons after traumatic brain injury in rats. Neurol Res 34:400–407. doi:10.1179/1743132812y.0000000025
Liu H, Liu G, Bi Y (2014) CNTF regulates neurite outgrowth and neuronal migration through JAK2/STAT3 and PI3K/Akt signaling pathways of DRG explants with gp120-induced neurotoxicity in vitro. Neurosci Lett 569:110–115. doi:10.1016/j.neulet.2014.03.071
Liu Y, Zhang Y, Lin L, Lin F, Li T, Du H, Chen R, Zheng W, Liu N (2013) Effects of bone marrow-derived mesenchymal stem cells on the axonal outgrowth through activation of PI3K/AKT signaling in primary cortical neurons followed oxygen-glucose deprivation injury. PLoS One 8:e78514. doi:10.1371/journal.pone.0078514
Perez-Alvarez MJ, Mateos L, Alonso A, Wandosell F (2014) Estradiol and progesterone administration after pMCAO stimulates the neurological recovery and reduces the detrimental effect of ischemia mainly in hippocampus. Mol Neurobiol. doi:10.1007/s12035-014-8963-7
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
M.-R. Chen, P. Dai, T.-H. Wang and J. Liu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, MR., Dai, P., Wang, SF. et al. BDNF Overexpression Exhibited Bilateral Effect on Neural Behavior in SCT Mice Associated with AKT Signal Pathway. Neurochem Res 41, 2585–2597 (2016). https://doi.org/10.1007/s11064-016-1970-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1970-5