Abstract
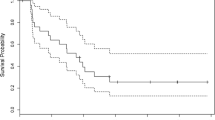
One resistance mechanism in malignant gliomas (MG) involves nuclear factor-κB (NF-κB) activation. Bortezomib prevents proteasomal degradation of NF-κB inhibitor α (NFKBIA), an endogenous regulator of NF-κB signaling, thereby limiting the effects of NF-κB on tumor survival and resistance. A presurgical phase II trial of bortezomib in recurrent MG was performed to determine drug concentration in tumor tissue and effects on NFKBIA. Patients were enrolled after signing an IRB approved informed consent. Treatment was bortezomib 1.7 mg/m2 IV on days 1, 4 and 8 and then surgery on day 8 or 9. Post-operatively, treatment was Temozolomide (TMZ) 75 mg/m2 PO on days 1–7 and 14–21 and bortezomib 1.7 mg/m2 on days 7 and 21 [1 cycle was (1) month]. Ten patients were enrolled (8 M and 2 F) with 9 having surgery. Median age and KPS were 50 (42–64) and 90 % (70–100). The median cycles post-operatively was 2 (0–4). The trial was stopped as no patient had a PFS-6. All patients are deceased. Paired plasma and tumor bortezomib concentration measurements revealed higher drug concentrations in tumor than in plasma; NFKBIA protein levels were similar in drug-treated vs. drug-naïve tumor specimens. Nuclear 20S proteasome was less in postoperative samples. Postoperative treatment with TMZ and bortezomib did not show clinical activity. Bortezomib appears to sequester in tumor but pharmacological effects on NFKBIA were not seen, possibly obscured due to downregulation of NFKBIA during tumor progression. Changes in nuclear 20S could be marker of bortezomib effect on tumor.


Similar content being viewed by others
References
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. doi:10.1056/NEJMoa043331
Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, Armstrong TS, Wefel JS, Won M, Blumenthal DT, Mahajan A, Schultz CJ, Erridge S, Baumert B, Hopkins KI, Tzuk-Shina T, Brown PD, Chakravarti A, Curran WJ Jr, Mehta MP (2013) Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 31:4085–4091. doi:10.1200/JCO.2013.49.6968
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740. doi:10.1200/JCO.2008.19.8721
Friedman HS, Kerby T, Calvert H (2000) Temozolomide and treatment of malignant glioma. Clin Cancer Res 6:2585–2597
Gerson SL (2002) Clinical relevance of MGMT in the treatment of cancer. J Clin Oncol 20:2388–2399
Pegg AE (2000) Repair of O(6)-alkylguanine by alkyltransferases. Mutat Res 462:83–100
Belanich M, Pastor M, Randall T, Guerra D, Kibitel J, Alas L, Li B, Citron M, Wasserman P, White A, Eyre H, Jaeckle K, Schulman S, Rector D, Prados M, Coons S, Shapiro W, Yarosh D (1996) Retrospective study of the correlation between the DNA repair protein alkyltransferase and survival of brain tumor patients treated with carmustine. Cancer Res 56:783–788
Friedman HS, McLendon RE, Kerby T, Dugan M, Bigner SH, Henry AJ, Ashley DM, Krischer J, Lovell S, Rasheed K, Marchev F, Seman AJ, Cokgor I, Rich J, Stewart E, Colvin OM, Provenzale JM, Bigner DD, Haglund MM, Friedman AH, Modrich PL (1998) DNA mismatch repair and O6-alkylguanine-DNA alkyltransferase analysis and response to temodal in newly diagnosed malignant glioma. J Clin Oncol 16:3851–3857
Jaeckle KA, Eyre HJ, Townsend JJ, Schulman S, Knudson HM, Belanich M, Yarosh DB, Bearman SI, Giroux DJ, Schold SC (1998) Correlation of tumor O6 methylguanine-DNA methyltransferase levels with survival of malignant astrocytoma patients treated with bis-chloroethylnitrosourea: a Southwest Oncology Group study. J Clin Oncol 16:3310–3315
Tolcher AW, Gerson SL, Denis L, Geyer C, Hammond LA, Patnaik A, Goetz AD, Schwartz G, Edwards T, Reyderman L, Statkevich P, Cutler DL, Rowinsky EK (2003) Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer 88:1004–1011. doi:10.1038/sj.bjc.66008276600827
Perry JR, Bélanger K, Mason WP, Fulton D, Kavan P, Easaw J, Shields C, Kirby S, Macdonald DR, Eisenstat DD, Thiessen B, Forsyth P, Pouliot JF (2010) Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol 28:2051–2057. doi:10.1200/JCO.2009.26.5520
Wick W, Platten M, Weller M (2009) New (alternative) temozolomide regimens for the treatment of glioma. Neuro-oncology 11:69–79. doi:10.1215/15228517-2008-078
Milano A, Iaffaioli RV, Caponigro F (2007) The proteasome: a worthwhile target for the treatment of solid tumours? Eur J cancer 43:1125–1133. doi:10.1016/j.ejca.2007.01.038
Conti A, Ageunnouz M, La Torre D, Cardali S, Angileri FF, Buemi C, Tomasello C, Iacopino DG, D’Avella D, Vita G, Tomasello F (2005) Expression of the tumor necrosis factor receptor-associated factors 1 and 2 and regulation of the nuclear factor-kappaB antiapoptotic activity in human gliomas. J Neurosurg 103:873–881
Weaver KD, Yeyeodu S, Cusack JC Jr, Baldwin AS Jr, Ewend MG (2003) Potentiation of chemotherapeutic agents following antagonism of nuclear factor kappa B in human gliomas. J Neurooncol 61:187–196
Bredel M, Bredel C, Juric D, Duran GE, Yu RX, Harsh GR, Vogel H, Recht LD, Scheck AC, Sikic BI (2006) Tumor necrosis factor-alpha-induced protein 3 as a putative regulator of nuclear factor-kappaB-mediated resistance to O6-alkylating agents in human glioblastomas. J Clin Oncol 24:274–287. doi:10.1200/JCO.2005.02.9405
Lavon I, Fuchs D, Zrihan D, Efroni G, Zelikovitch B, Fellig Y, Siegal T (2007) Novel mechanism whereby nuclear factor kappaB mediates DNA damage repair through regulation of O(6)-methylguanine-DNA-methyltransferase. Cancer Res 67:8952–8959. doi:10.1158/0008-5472.CAN-06-3820
Bredel M, Scholtens DM, Yadav AK, Alvarez AA, Renfrow JJ, Chandler JP, Yu IL, Carro MS, Dai F, Tagge MJ, Ferrarese R, Bredel C, Phillips HS, Lukac PJ, Robe PA, Weyerbrock A, Vogel H, Dubner S, Mobley B, He X, Scheck AC, Sikic BI, Aldape KD, Chakravarti A, Harsh GRt (2011) NFKBIA deletion in glioblastomas. N Engl J Med 364:627–637. doi:10.1056/NEJMoa1006312
Vlachostergios PJ, Hatzidaki E, Stathakis NE, Koukoulis GK, Papandreou CN (2013) Bortezomib downregulates MGMT expression in T98G glioblastoma cells. Cell Mol Neurobiol 33:313–318. doi:10.1007/s10571-013-9910-2
Richardson PG, Hideshima T, Anderson KC (2003) Bortezomib (PS-341): a novel, first-in-class proteasome inhibitor for the treatment of multiple myeloma and other cancers. Cancer Control J Moffitt Cancer Center 10:361–369
McConkey DJ, Zhu K (2008) Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resistance Updates Rev Comment Antimicrob Anticancer Chemother 11:164–179 doi:10.1016/j.drup.2008.08.002
Bu R, Hussain AR, Al-Obaisi KA, Ahmed M, Uddin S, Al-Kuraya KS (2014) Bortezomib inhibits proteasomal degradation of IkappaBalpha and induces mitochondrial dependent apoptosis in activated B-cell diffuse large B-cell lymphoma. Leukemia Lymphoma 55:415–424 doi:10.3109/10428194.2013.806799
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Kubicek GJ, Werner-Wasik M, Machtay M, Mallon G, Myers T, Ramirez M, Andrews D, Curran WJ Jr, Dicker AP (2009) Phase I trial using proteasome inhibitor bortezomib and concurrent temozolomide and radiotherapy for central nervous system malignancies. Int J Radiat Oncol Biol Phys 74:433–439. doi:10.1016/j.ijrobp.2008.08.050
Phuphanich S, Supko JG, Carson KA, Grossman SA, Burt Nabors L, Mikkelsen T, Lesser G, Rosenfeld S, Desideri S, Olson JJ (2010) Phase 1 clinical trial of bortezomib in adults with recurrent malignant glioma. J Neurooncol 100:95–103. doi:10.1007/s11060-010-0143-7
Portnow J, Frankel P, Koehler S, Twardowski P, Shibata S, Martel C, Morgan R, Cristea M, Chow W, Lim D, Chung V, Reckamp K, Leong L, Synold TW (2012) A phase I study of bortezomib and temozolomide in patients with advanced solid tumors. Cancer Chemother Pharmacol 69:505–514. doi:10.1007/s00280-011-1721-x
Friday BB, Anderson SK, Buckner J, Yu C, Giannini C, Geoffroy F, Schwerkoske J, Mazurczak M, Gross H, Pajon E, Jaeckle K, Galanis E (2012) Phase II trial of vorinostat in combination with bortezomib in recurrent glioblastoma: a north central cancer treatment group study. Neuro-oncology 14:215–221. doi:10.1093/neuonc/nor198
Odia Y, Kreisl TN, Aregawi D, Innis EK, Fine HA (2015) A phase II trial of tamoxifen and bortezomib in patients with recurrent malignant gliomas. J Neurooncol 125:191–195. doi:10.1007/s11060-015-1894-y
Vilas-Boas Fde A, da Silva AM, de Sousa LP, Lima KM, Vago JP, Bittencourt LF, Dantas AE, Gomes DA, Vilela MC, Teixeira MM, Barcelos LS (2016) Impairment of stress granule assembly via inhibition of the eIF2alpha phosphorylation sensitizes glioma cells to chemotherapeutic agents. J Neurooncol 127:253–260. doi:10.1007/s11060-015-2043-3
Yoo JY, Hurwitz BS, Bolyard C, Yu JG, Zhang J, Selvendiran K, Rath KS, He S, Bailey Z, Eaves D, Cripe TP, Parris DS, Caligiuri MA, Yu J, Old M, Kaur B (2014) Bortezomib-induced unfolded protein response increases oncolytic HSV-1 replication resulting in synergistic antitumor effects. Clin Cancer Res 20:3787–3798. doi:10.1158/1078-0432.CCR-14-0553
Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ (1999) Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res 59:2615–2622
Reece DE, Sullivan D, Lonial S, Mohrbacher AF, Chatta G, Shustik C, Burris H 3rd, Venkatakrishnan K, Neuwirth R, Riordan WJ, Karol M, von Moltke LL, Acharya M, Zannikos P, Keith Stewart A (2011) Pharmacokinetic and pharmacodynamic study of two doses of bortezomib in patients with relapsed multiple myeloma. Cancer Chemother Pharmacol 67:57–67. doi:10.1007/s00280-010-1283-3
Labussiere M, Pinel S, Delfortrie S, Plenat F, Chastagner P (2008) Proteasome inhibition by bortezomib does not translate into efficacy on two malignant glioma xenografts. Oncol Rep 20:1283–1287
Chen D, Frezza M, Schmitt S, Kanwar J, Dou QP (2011) Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Curr Cancer Drug Targets 11:239–253
Muralidharan S, Mandrekar P (2013) Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukoc Biol 94:1167–1184. doi:10.1189/jlb.0313153
Acknowledgments
We thank Michael Avram, PhD for this review of the manuscript and comments on the drug distribution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has a conflict of interest related to this manuscript.
Additional information
This trial was supported by Millennium Pharmaceuticals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Raizer, J.J., Chandler, J.P., Ferrarese, R. et al. A phase II trial evaluating the effects and intra-tumoral penetration of bortezomib in patients with recurrent malignant gliomas. J Neurooncol 129, 139–146 (2016). https://doi.org/10.1007/s11060-016-2156-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2156-3