Abstract
Protocells are supposed to have played a key role in the self-organizing processes leading to the emergence of life. Existing models either (i) describe protocell architecture and dynamics, given the existence of sets of collectively self-replicating molecules for granted, or (ii) describe the emergence of the aforementioned sets from an ensemble of random molecules in a simple experimental setting (e.g. a closed system or a steady-state flow reactor) that does not properly describe a protocell. In this paper we present a model that goes beyond these limitations by describing the dynamics of sets of replicating molecules within a lipid vesicle. We adopt the simplest possible protocell architecture, by considering a semi-permeable membrane that selects the molecular types that are allowed to enter or exit the protocell and by assuming that the reactions take place in the aqueous phase in the internal compartment. As a first approximation, we ignore the protocell growth and division dynamics. The behavior of catalytic reaction networks is then simulated by means of a stochastic model that accounts for the creation and the extinction of species and reactions. While this is not yet an exhaustive protocell model, it already provides clues regarding some processes that are relevant for understanding the conditions that can enable a population of protocells to undergo evolution and selection.





Similar content being viewed by others
Notes
Limitations on the outflow can be modeled in a chemostat e.g. by supposing that all the molecules that are larger than a certain size precipitate and cannot be washed away.
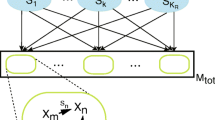
It is worthwhile to notice that the presence of the“ catalysis” within the tuple allows the possibility for a species to catalyze more than one reaction and for a reaction to be catalyzed by more than one species.
Exceptionally for the case 1 μM, \(\Theta \) is not computed after 3,000 s but when at least 5,000 reactions have occurred within the simulation. The reason for this is that the low concentrations involve a so slow dynamics that 3,000 s are not enough in order to observe significant chemical changes.
In regard to CH2 we are here considering the value of \(\Theta \) excluding the species belonging to the RAF set (last columns of the table). Since the molecules belonging to the RAF set reach a concentration much greater with respect to the other molecules, considering them in the angle computation would misrepresent the distance among the simulations.
We set the sampling frequency and the time threshold of the windows by taking advantage from several initial model threads, not essential to the comprehension of this article.
This property was proved earlier by Munteanu et al. for the Los Alamos bug mode (Munteanu et al. 2007).
References
Alessandro F, Graudenzi A, Serra R, Villani M, Füchslin RM, Packard N, Kauffman S a, Poli I (2011) A stochastic model of autocatalytic reaction networks. Theory in biosciences = Theorie in den Biowissenschaften, p 1–9, October 2011
Bagley RJ, Farmer JD (1992) Spontaneous emergence of a metabolism. Artificial Life II (Santa Fe Institute Studies in the Sciences of Complexity) 10:93–141
Cans A-S, Andes-Koback M, Keating CD (2008) Positioning lipid membrane domains in giant vesicles by micro-organization of aqueous cytoplasm mimic. J Am Chem Soc 130(23):7400–7406
Carletti T, Serra R, Villani M, Poli I, Filisetti A (2008) Sufficient conditions for emergent synchronization in protocell models. J Theor Biol 254(4):741–751
Carletti T, Filisetti A (2012) The stochastic evolution of a protocell: The Gillespie algorithm in a dynamically varying volume. Comput Math Methods Med 2012(Article ID 423627):1–13
Dai X, Yli-Harja O, Ribeiro AS (2009) Determining noisy attractors of delayed stochastic gene regulatory networks from multiple data sources. Bioinform (Oxf, Engl) 25(18):8–2362
de Souza TP, Stano P, Steiniger F, D’Aguanno E, Altamura E, Fahr A, Luigi LP (2012) Encapsulation of ferritin, ribosomes, and ribo-peptidic complexes inside liposomes: insights into the origin of metabolism. Origins Life Evol Biosph 42(5):421–428
Dominak LM, Keating CD (2007) Polymer encapsulation within giant lipid vesicles. Langmuir 23(13):7148–7154
Dyson FJ (1985) Origins of life. Cambridge University Press, Cambridge
Eigen M, Schuster P (1977) The hypercycle. A principle of natural self-organization. Part A: emergence of the hypercycle. Die Naturwissenschaften 64(11):65–541
Farmer JD, Kauffman SA (1986) Autocatalytic replication of polymers. Phys D: Nonlinear Phenom 220:50–67
Filisetti A, Graudenzi A, Serra R, Villani M, De Lucrezia D, Poli I (2011) The role of energy in a stochastic model of the emergence of autocatalytic sets. In: Lenaerts T, Giacobini M, Bersini H, Bourgine P, Dorigo M, Doursat R (eds) Advances in artificial life, ECAL 2011 proceedings of the eleventh European conference on the synthesis and simulation of living systems. MIT Press, Cambridge, pp 227–234
Filisetti A, Serra R, Carletti T, Villani M, Poli I (2010) Non-linear protocell models: synchronization and chaos. Eur Phys J B 77(2):249–256
Filisetti A, Graudenzi A, Serra R, Villani M, De Lucrezia D, Fuchslin RM, Kauffman SA, Packard N, Poli I (2011) A stochastic model of the emergence of autocatalytic cycles. J Syst Chem 2(1):2
Ganti T (2003) Chemoton theory, Vol I: theory of fluyd machineries and Vol II: theory of livin system. Kluwer Academic/Plenum, New York
Gillespie DT (1977) Exact stochastic simulation of coupled chemical reactions. J Phys Chem 81(25):2340–2361
Gillespie DT (2007) Stochastic simulation of chemical kinetics. Ann Rev Phys Chem 58(1):35–55
Hanczyc MM, Fujikawa SM, Szostak JW (2003) Experimental models of primitive cellular compartments: encapsulation, growth, and division. Sci (N Y) 302(5645):618–622
Hordijk W, Fontanari JF (2002) International Conference on IEEE catalytic reaction sets decay, and the preservation of information p 133–138
Hordijk W, Steel M (2004) Detecting autocatalytic, self-sustaining sets in chemical reaction systems. J Theor Biol 227(4):451–461
Hordijk W, Hein J, Steel M (2010) Autocatalytic sets and the origin of life. Entropy 12(7):1733–1742
Hordijk W, Steel M, Kauffman S (2012) The structure of autocatalytic sets: evolvability, enablement, and emergence. Acta Biotheor 60(4):379–392
Jain S, Krishna S (1998) Autocatalytic set and the growth of complexity in an evolutionary model. Phys Rev Lett 81:5684–5687
Jain S, Krishna S (2001) A model for the emergence of cooperation, interdependence, and structure in evolving networks. Proc Natl Acad Sci USA 98(2):543–547
Kaneko K (2006) Life: an introduction to complex systems biology (understanding complex systems). Springer-Verlag New York Inc, Secaucus
Kauffman SA (1986) Autocatalytic sets of proteins. J Theor Biol 119(1):1–24
Luisi PL, Ferri F, Stano P (2006) Approaches to semi-synthetic minimal cells: a review. Naturwissenschaften 93(1):1–13
Mansy SS (2009) Model protocells from single-chain lipids. Int J Mol Sci 10(3):835–843
Mansy SS, Schrum JP, Krishnamurthy M, Tobé S, Treco DA, Szostak JW (2008) Template-directed synthesis of a genetic polymer in a model protocell. Nature 454(7200):122–125
Morowitz HJ, Heinz B, Deamer DW (1988) The chemical logic of a minimum protocell. Origins Life Evol Bios 18(3):281–287
Morris SC (2003) Life’s solution. Inevitable humans in a lonely Universe. University of Cambridge, Cambridge
Mouritsen OG (2005) Life—as a matter of fat. The emerging science of lipidomics, 1st edn. Springer, Berlin
Munteanu A, Attolini CS, Rasmussen S, Ziock H, Solé RV (2007) Generic Darwinian selection in catalytic protocell assemblies. Phil Trans R Soc B 362:1847–1855
Munteanu A, Solé RV (2006) Phenotypic diversity and chaos in a minimal cell model. J Theor Biol 240(3):434–442
Polozova A, Li X, Shangguan T, Meers P, Schuette DR, Ando N, Gruner SM, Perkins WR (2005) Formation of homogeneous unilamellar liposomes from an interdigitated matrix. Biochim et Biophys Acta 1668(1):117–125
Rasmussen S, Chen L, Deamer D, Krakauer DC, Packard NH, Stadler PF, Bedau MA (2004) Transitions from nonliving to living matter. Science 303:963–965
Rasmussen S, Chen L, Nilsson M, Abe S (2003) Bridging nonliving and living matter. Artif Life 9(3):269–316
Rocheleau T, Rasmussen S, Nielsen PE, Jacobi MN (2007) Emergence of protocellular growth laws. Philos Trans R Soc Lond B Biol Sci 362(1486):1841–1845
Segré D, Lancet D (2000) Composing life. EMBO Rep 1(3):22–217
Serra R, Carletti T, Poli I (2007) Syncronization phenomena in surface-reaction models of protocells. Artif Life 123(2):123–138
Serra R, Carletti T, Poli I, Villani M, Filisetti A (2007) Conditions for emergent synchronization in protocell. In In J Jost and D Helbing (eds): Proceedings of ECCS07: European Conference on Complex Systems. CD-Rom, paper no. 68
Serra R, Filisetti A, Villani M, Damiani C, Graudenzi A, Panini T (2013) A stochastic model of catalytic reaction networks in protocells. Artif Life
Serra R, Villani M (2013) Mechanism for the formation of density gradients through semipermeable membranes. Phys Rev E 87(6):062814
Ricard RV, Solé V, Munteanu A, Rodriguez-Caso C, Macía J (2007) Synthetic protocell biology: from reproduction to computation. Philos Trans R Soc Lond B Biol Sci 362(1486):1727–1739
Stadler PF (1991) Dynamics of autocatalytic reaction networks. IV: inhomogeneous replicator networks. Bio Syst 26(1):1–19
Stadler PF, Schuster P (1990) Dynamics of small autocatalytic reaction networks-I. Bifurcations, permanence and exclusion. Bulletin Math Biol 52(4):485–508
Stadler PF, Schnabl W, Forst C, Schuster P (1995) Dynamics of small autocatalytic reaction networks II replication, mutation and catalysis. Bulletin Math Biol 57(1):21–61
Stano P, Luigi Luisi P (2010) Achievements and open questions in the self-reproduction of vesicles and synthetic minimal cells. Chem Commun (Camb, Engl) 46(21):53–3639
Szostak JW, Bartel DP, Luisi PL (2001) Synthesizing life. Nature 409(6818):387–390
Vasas V, Fernando C, Santos M, Kauffman S, Szathmary E (2012) Evolution before genes. Biol Direct 7(1):1
Wesson R (1991) Beyond natural selection. MIT Press, Cambridge
Zhu TF, Szostak JW (2009) Coupled growth and division of model protocell membranes. J Am Chem Soc 131(15):5705–5713
Acknowledgments
Stuart Kauffman, Norman Packard and Wim Hordijk kindly shared with us their deep understanding of autocatalytic sets in several useful discussions. Useful discussions with Ruedi Füchslin, Davide De Lucrezia, Timoteo Carletti, Andrea Roli and Giulio Caravagna are also gratefully acknowledged. The authors are also grateful to Giulia Begal for kindly drawing the image of Fig. 2. C.D. wishes to acknowledge the project SysBionet (12-4-5148000-15; Imp. 611/12; CUP: H41J12000060001; U.A. 53) for the financial support of the work.
Author information
Authors and Affiliations
Corresponding author
Appendix 1: Simulation environment and parameter settings
Appendix 1: Simulation environment and parameter settings
Simulations were performed with the CaRNeSS simulatorFootnote 8 developed by the research group.
In the following, the baseline setting of the system used in the simulations is reported (for the parameters that were variated in the different experiments please refer to the text): \((\bullet )\) Alphabet: A, B, \((\bullet )\) Volume = \( = 1e-18 dm^3 = 1{\rm \mu} ^3\), \((\bullet )\) Average catalysis probability = 1 catalyzed reaction for species, \((\bullet )\) Maximum length of the species, \(L_{max} = 6\), \((\bullet )\) \(L_{perm} = 2\), \((\bullet )\) Monomers and dimers do NOT catalyze, \((\bullet )\) \(K_{cleav}= 25M^{-1}sec^{-1}\), \((\bullet )\) \(K_{comp}=50M^{-1}sec^{-1}\), \((\bullet )\) \(K_{diss}=1M^{-1}sec^{-1}\), \((\bullet )\) \(K_{cond}=50M^{-1}sec^{-1}\).
Rights and permissions
About this article
Cite this article
Serra, R., Filisetti, A., Villani, M. et al. A stochastic model of catalytic reaction networks in protocells. Nat Comput 13, 367–377 (2014). https://doi.org/10.1007/s11047-014-9445-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11047-014-9445-6