Abstract
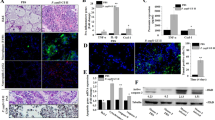
Prototheca zopfii is an important bovine mastitis pathogen, which could result in severe mammary infection. However, the innate immune response in bovine mastitis associated with P. zopfii was not clear. Therefore, the aim of this study is to investigate in vitro innate immune responses implicated by P. zopfii. Bovine mammary epithelial cells (bMECs) were infected with 5.0 × 104 cells/ml P. zopfii genotypes I and II independently, and the mRNA expression of TLR-2, TLR-4, TNF-α, IL-1β, IL-8, NOD-1, NOD-2 and β-defensin-5 was determined by real-time polymerase chain reaction (RT-PCR) over a time course of 1, 3, 6, 12 and 24 h. The detection of the NF-κB p65 protein in nucleus and cytoplasm of infected bMECs over the same time course was evaluated. Results showed that P. zopfii genotype II has ability to up-regulate the expression of TLR-2, TLR-4, TNF-α, IL-1β, IL-8, NOD-1, NOD-2 and β-defensin-5 'more strongly than genotype I. Western blot results showed that when bMECs were challenged by P. zopfii genotype II, the expression of NF-κB p65 protein in the nucleus was up-regulated, while in cytoplasm it appeared to be repressed, which indicated that bMECs partly regulate the innate immune responses and inflammation by the NF-κB signaling pathway while being infected by P. zopfii genotype II. It was concluded that adhesion of genotype II was stronger than genotype I, and therefore the genotype II regulatory ability is more robust than that of the genotype I, which causes inflammation of bovine mammary tissue.









Similar content being viewed by others
References
Roesler U, Möller A, Hensel A, Baumann D, Truyen U. Diversity within the current algal species Prototheca zopfii: a proposal for two Prototheca zopfii genotypes and description of a novel species, Prototheca blaschkeae sp. nov. Int J Syst Evol Microbiol. 2006;56:1419–25.
Jagielski T, Lagneau PE. Protothecosis. A pseudofungal infection. J Mycol Med. 2007;17:261–70.
Lass-Florl C, Mayr A. Human protothecosis. Clin Microbiol Rev. 2007;20:230–42.
Macesic N, Fleming S, Kidd S, Madigan V, Chean R, Ritchie D, et al. Protothecosis in hematopoietic stem cell transplantation: case report and review of previous cases. Transpl Infect Dis. 2014;16:490–5.
Wawron W, Bochniarz M, Piech T, Łopuszyński W, Wysocki J. Outbreak of protothecal mastitis in a herd of dairy cows in Poland. Bull Vet Inst Pulawy. 2013;57:335–9.
Jagielski T, Lassa H, Ahrholdt J, Roesler U, Malinowski E. Molecular characterization of polish Prototheca zopfii mastitis isolates and first isolation of Prototheca blaschkeae in Poland. Pol J Vet Sci. 2010;13:725–9.
Gao J, Zhang HQ, He JZ, He YH, Li SM, Hou RG, et al. Characterization of Prototheca zopfii associated with outbreak of bovine clinical mastitis in herd of Beijing, China. Mycopathologia. 2012;173:275–81.
Shahid M, Ali T, Zhang L, Hou R, Zhang S, Ding L, Han D, et al. Characterization of Prototheca zopfii genotypes isolated from cases of bovine mastitis and cow barns in China. Mycopathologia. 2015;181:185–95.
Sordillo LM, Streicher KL. Mammary gland immunity and mastitis susceptibility. J Mam Gland Biol Neoplasia. 2002;7:135–46.
Alluwaimi AM. The cytokines of bovine mammary gland: prospects for diagnosis and therapy. Res Vet Sci. 2004;77:211–22.
Dion WM. Bovine mastitis due to Prototheca zopfii II. Can Vet J. 1982;23:272–5.
Roesler U, Scholz H, Hensel A. Immunodiagnostic identification of dairy cows infected with Prototheca zopfii at various clinical stages and discrimination between infected and uninfected cows. J Clin Microbiol. 2001;39:539–43.
Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216.
Roesler U, Hensel A. Longitudinal analysis of Prototheca zopfii-specific immune responses: correlation with disease progression and carriage in dairy cows. J Clin Microbiol. 2003;41:1181–6.
Chang R, Yang Q, Liu G, Liu Y, Zheng B, Su J, et al. Treatment with gentamicin on a murine model of Protothecal mastitis. Mycopathologia. 2013;175:241–8.
Schalm OW, Moorlander DO. Experiments and observations leading to the development of the California Mastitis test. J Am Vet Med Assoc. 1957;130:199–204.
Liu Y, Chen W, Ali T, Alkasir R, Yin J, Liu G, et al. Staphylococcal enterotoxin H induced apoptosis of bovine mammary epithelial cells in vitro. Toxins. 2014;6:3552–67.
Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucl Acids Res. 2002;30:36.
Fu Y, Zhou E, Liu Z, Li F, Liang D, Liu B, et al. Staphylococcus aureus and Escherichia coli elicit different innate immune responses from bovine mammary epithelial cells. Vet Immunol Immunopathol. 2013;155:245–52.
Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80.
Rabot A, Wellnitz O, Meyer HH, Bruckmaier RM. Use and relevance of a bovine mammary gland explant model to study infection responses in bovine mammary tissue. J Dairy Res. 2007;74:93–9.
Wellnitz O, Bruckmaier RM. The innate immune response of the bovine mammary gland to bacterial infection. Vet J. 2012;192:148–52.
Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388:621–5.
Murray PJ. NOD proteins: an intracellular pathogen-recognition system or signal transduction modifiers? Curr Opin Immunol. 2005;17:352–8.
Divangahi M, Mostowy S, Coulombe F, Kozak R, Guillot L, Veyrier F, et al. NOD2-deficient mice have impaired resistance to Mycobacterium tuberculosis infection through defective innate and adaptive immunity. J Immunol. 2008;181:7157–65.
Shaw MH, Reimer T, Sanchez-Valdepenas C, Warner N, Kim YG, Fresno M, et al. T cell-intrinsic role of Nod2 in promoting type1 immunity to Toxoplasma gondii. Nat Immunol. 2009;10:1267–74.
Moreira LO, Zam-boni DS. NOD1 and NOD2 signaling in infection and inflammation. Front Immunol. 2012;3:328.
Christman JW, Sadikot RT, Blackwell TS. The role of nuclear factor-kappa B in pulmonary diseases. Chest. 2000;117:1482–7.
McCoy MK, Ruhn KA, Blesch A, Tansey MG. TNF: a key neuroinflammatory mediator of neurotoxicity and neurodegeneration in models of Parkinson’s disease. Adv Exp Med Biol. 2011;691:539–40.
Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:12–26.
Gray GD, Knight KA, Nelson RD, Herron MJ. Chemotactic requirements of bovine leucocytes. Am J Vet Res. 1982;43:757–9.
Craven N. Chemotactic factors for bovine neutrophils in relation to mastitis. Comp Immunol Microbiol Infect Dis. 1986;9:29–36.
Baggiolini M, Dahinden CA. CC chemokines in allergic inflammation. Immunology. 1994;15:127–33.
Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–6.
Acknowledgments
This research was supported by the Chinese Twelfth ‘‘Five-year’’ National Science and Technology Support Project (No. 2012BAD12B03), National Education Ministry’s Major Project (No. 313054), Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP) State Education Ministry (No. 20120008110042), High-end Foreign Experts Recruitment Program (No. GDT20141100043) and the National Natural Science Foundation of China (No. 31572587).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Zhaoju Deng and Muhammad Shahid have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Deng, Z., Shahid, M., Zhang, L. et al. An Investigation of the Innate Immune Response in Bovine Mammary Epithelial Cells Challenged by Prototheca zopfii . Mycopathologia 181, 823–832 (2016). https://doi.org/10.1007/s11046-016-0053-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-016-0053-0