Abstract
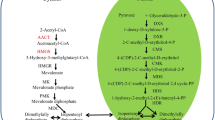
Thiolases are functionally divided into two groups: 3-ketoacyl-CoA thiolase and acetoacetyl-CoA thiolase (ACAT). Acetoacetyl-CoA thiolase plays a key role in the mevalonate pathway. In this study, a novel gene, IgACAT, which encodes ACAT was cloned from Isochrysis galbana and characterized. The cDNA of IgACAT was 1551 bp in length, consisting of an open reading frame of 1173 bp, a 5′ untranslated region of 69 bp and a 3′ untranslated region of 309 bp. The deduced amino acid sequence of IgACAT was 390 amino acid residues in length with a predicted molecular weight of 53.59 kDa and an isoelectric point of pH 9.04. The triterpenes content and the expression of IgACAT under nitrogen and temperature stress were analyzed. When I. galbana was treated with excessive nitrogen and at 35 °C, respectively, both the triterpenes content and the abundance of IgACAT gene transcript increased. Our findings will facilitate the regulation of gene expression and genetic modification of the triterpenes synthesis pathway of I. galbana.



Similar content being viewed by others
References
Berges T, Guyonnet D, Karst F (1997) The Saccharomyces cerevisiae mevalonate diphosphate decarboxylase is essential for viability, and a single Leu-to-Pro mutation in a conserved sequence leads to thermosensitivity. J Bacteriol 179(15):4664–4670
Modis Y, Wierenga RK (2000) Crystallographic analysis of the reaction pathway of Zoogloea ramigera biosynthetic thiolase. J Mol Biol 297(5):1171–1182
Kursula P, Ojala J, Lambeir AM, Wierenga RK (2002) The catalytic cycle of biosynthetic thiolase: a conformational journey of an acetyl group through four binding modes and two oxyanion holes. Biochemistry 41(52):15543–15556
Clinkenbeard KD, Sugiyama T, Moss J, Reed WD, Lane MD (1973) Molecular and catalytic properties of cytosolic acetoacetyl coenzyme A thiolase from avian liver. J Biol Chem 248(7):2275–2284
Ren A, Qin L, Shi L, Dong X, Mu DS, Li YX, Zhao MW (2010) Methyl jasmonate induces ganoderic acid biosynthesis in the basidiomycetous fungus Ganoderma lucidum. Bioresour Technol 101(17):6785–6790
Liang CX, Li YB, Xu JW, Wang JL, Miao XL, Tang YJ, Gu T, Zhong JJ (2010) Enhanced biosynthetic gene expressions and production of ganoderic acids in static liquid culture of Ganoderma lucidum under phenobarbital induction. Appl Microbiol Biotechnol 86(5):1367–1374
Fang X, Shi L, Xu YJ, Zhao MW (2011) Cloning of a sterol 14α-demethylase gene and the effects of over-expression of the gene on biological synthesis of triterpenes in Ganoderma lucidum. Mycosystema 30(2):242–248
Shi L, Ren A, Mu D, Zhao M (2010) Current progress in the study on biosynthesis and regulation of ganoderic acids. Appl Microbiol Biotechnol 88(6):1243–1251
Harrison PJ, Waters RE, Taylor F (1980) A broad spectrum artificial sea water medium for coastal and open ocean phytoplankton. J Phycol 16(1):28–35
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408
Tang YJ, Zhong JJ (2002) Fed-batch fermentation of Ganoderma lucidum for hyperproduction of polysaccharide and ganoderic acid. Enzyme and Microbial Technology 31(1):20–28
Na L, Liu XH, Zhou J, Li YX, Zhao MW (2006) Analysis of influence of environmental conditions on ganoderic acid content in Ganoderma lucidum using orthogonal design. J Microbiol Biotechnol 16(12):1940–1946
Dyer JH, Maina A, Gomez ID, Cadet M, Oeljeklaus S, Schiedel AC (2009) Cloning, expression and purification of an acetoacetyl CoA thiolase from sunflower cotyledon. Int J bio sci 5(7):736
Harris JI, Gehring U (1970) The active site cysteines of thiolase. Eur J Biochem 16(3):492–498
Williams SF, Palmer M, Peoples O, Walsh C, Sinskey A, Masamune S (1992) Biosynthetic thiolase from Zoogloea ramigera. Mutagenesis of the putative active-site base Cys-378 to Ser-378 changes the partitioning of the acetyl S-enzyme intermediate. J Biol Chem 267(23):16041–16043
Vollack KU, Bach TJ (1996) Cloning of a cDNA encoding cytosolic acetoacetyl-coenzyme A thiolase from radish by functional expression in Saccharomyces cerevisiae. Plant Physiol 111(4):1097–1107
Xu JW, Xu YN, Zhong JJ (2010) Production of individual ganoderic acids and expression of biosynthetic genes in liquid static and shaking cultures of Ganoderma lucidum. Appl Microbiol Biotechnol 85(4):941–948
Zhang WX, Tang YJ, Zhong JJ (2010) Impact of oxygen level in gaseous phase on gene transcription and ganoderic acid biosynthesis in liquid static cultures of Ganoderma lucidum. Bioprocess Biosyst Eng 33(6):683–690
Acknowledgments
This work was supported by Provincial Natural Science Foundation of Shandong (2010ZRA02013), National Natural Science Foundation of China (41106148) and Ocean Industry Research Special Project (200905019).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, Y., Zheng, M., Wan, W. et al. Effect of nitrogen and temperature on the transcription of an ACAT gene in Isochrysis galbana . Mol Biol Rep 41, 7235–7240 (2014). https://doi.org/10.1007/s11033-014-3608-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3608-5