Abstract
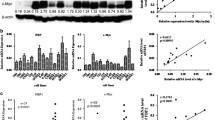
To determine the expression and function of B cell translocation gene 1 (BTG1) in nasopharyngeal carcinoma. Nasopharyngeal samples were taken from cancer lesions (n = 75) and adjacent normal tissue (n = 33) in nasopharyngeal cancer patients immediately after endoscopic biopsy. BTG1 expression was determined by immunohistochemistry and Western blotting. The effect of BTG1 overexpression was examined in vitro utilizing a human nasopharyngeal cancer cell line CNE2 stably transfected with a recombinant lentivirus (LeBTG1 cells) and compared to empty vector-transfected controls (LeEmpty). BTG1 overexpression was verified by real-time reverse transcriptase polymerase chain reaction and Western blot. The expression of proteins involved in cell cycle regulation (cyclin D1), apoptosis (Bcl-2) and cell migration (MMP-9) in LeBTG1 cells were analyzed by Western blot. The effect of BTG1 overexpression on cell viability and proliferation was assessed by an MTT assay in LeBTG1 and LeEmpty cells. Flow cytometric analyses were used to evaluate the effect of BTG1 expression on cell cycle distribution and apoptosis. The migration and invasion potential of LeBTG1 cells was examined by plating cells in Matrigel-coated chambers. BTG1 protein expression was significantly lower in nasopharyngeal cancer tissue biopsies than normal tissue as measured by immunohistochemistry (36.0 vs. 81.8 % of tissues; P < 0.05) and Western blotting (0.221 ± 0.019 vs. 0.652 ± 0.055; P < 0.05). Decreased expression of BTG1 was significantly correlated with nasopharyngeal cancer tumor stage, lymph node metastasis, clinical stage and pathologic differentiation (P < 0.05), as well as with reduced overall five-year survival rates compared to patients with higher expression levels (31.2 vs. 70.2 %; P < 0.05). In vitro analyses revealed that LeBTG1 cells had a reduced survival fraction compared to control LeEmpty cells, with higher rates of apoptosis (9.3 ± 0.7 vs. 2.3 ± 0.3 %; P < 0.05). The proportion of LeBTG1 cells in G0/G1 stage and S phase was also significantly different from LeEmpty cells (82.6 ± 3.8 and 10.1 ± 1.0 %, vs. 62.2 ± 2.4 and 28.9 ± 2.0 %, respectively; Ps < 0.05), and the migration and invasion of LeBTG1 cells was significantly impaired with respect to LeEmpty cells (96.0 ± 13.0 and 91.0 ± 11.0 vs. 158.0 ± 17.0 and 142.0 ± 15.0, respectively; Ps < 0.05). These effects were accompanied by decreased protein expression of cyclin D1, Bcl-2 and MMP-9 in LeBTG1 cells (0.231 ± 0.021, 0.413 ± 0.046, 0.131 ± 0.011, respectively) compared to control LeEmpty cells (0.636 ± 0.067, 0.821 ± 0.083, 0.451 ± 0.041, respectively; Ps < 0.05). Reduced BTG1 expression is associated with increased disease severity, suggesting it is a negative regulator of nasopharyngeal cancer and can serve as a prognostic indicator.









Similar content being viewed by others
References
Agathocleous M, Harris WA (2013) Metabolism in physiological cell proliferation and differentiation. Trends Cell Biol 23:484–492
Hofmockel G (2000) Molecular genetic principles of tumor development and progression. Urologe A 39:212–213
Shibata D, Aaltonen LA (2001) Genetic predisposition and somatic diversification in tumor development and progression. Adv Cancer Res 80:83–114
Lee EY, Muller WJ (2010) Oncogenes and tumor suppressor genes. Cold Spring Harb Perspect Biol 2:a003236
Okuyama T, Maehara Y, Kabashima A, Takahashi I, Kakeji Y, Sugimachi K (2002) Combined evaluation of expressions of p53 and p21 proteins as prognostic factors for patients with gastric carcinoma. Oncology 63:353–361
Vadgama JV, Scuric Z, Chakrabarti R, Marzo E, Shen D, Wu Y (2006) Insulin-like growth factor I differentially regulates the expression of HIRF1/hCAF1 and BTG1 genes in human MCF-7 breast cancer cells. Int J Mol Med 18:129–139
Cortes U, Moyret-Lalle C, Falette N, Duriez C, Ghissassi FE, Barnas C, Morel AP, Hainaut P, Magaud JP, Puisieux A (2000) BTG gene expression in the p53-dependent and -independent cellular response to DNA damage. Mol Carcinog 27:57–64
Winkler GS (2010) The mammalian anti-proliferative BTG/Tob protein family. J Cell Physiol 222:66–72
Rouault JP, Rimokh R, Tessa C, Paranhos G, Ffrench M, Duret L, Garoccio M, Germain D, Samarut J, Magaud JP (1992) BTG1, a member of a new family of antiproliferative genes. EMBO J 11:1663–1670
Rouault JP, Falette N, Guéhenneux F, Guillot C, Rimokh R, Wang Q, Berthet C, Moyret-Lalle C, Savatier P, Pain B, Shaw P, Berger R, Samarut J, Magaud JP, Ozturk M, Samarut C, Puisieux A (1996) Identification of BTG2, an antiproliferative p53-dependent component of the DNA damage cellular response pathway. Nat Genet 14:482–486
Matsuda S, Rouault J, Magaud J, Berthet C (2001) In search of a function for the TIS21/PC3/BTG1/TOB family. FEBS Lett 497:67–72
Bozec A, Peyrade F, Milano G (2013) Molecular targeted therapies in the management of head and neck squamous cell carcinoma: recent developments and perspectives. Anticancer Agents Med Chem 13:389–402
Suzuki K, Nakamura K, Kato K, Hamada H, Tsukamoto T (2007) Exploration of target molecules for prostate cancer gene therapy. Prostate 67:1163–1173
Turashvili G, Bouchal J, Ehrmann J, Fridman E, Skarda J, Kolar Z (2007) Novel immunohistochemical markers for the differentiation of lobular and ductal invasive breast carcinomas. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 151:59–64
Ranganathan V, De PK (1996) Western blot of proteins from Coomassie-stained poly-acrylamide gels. Anal Biochem 234:102–104
van Meerloo J, Kaspers GJ, Cloos J (2011) Cell sensitivity assays: the MTT assay. Methods Mol Biol 731:237–245
Rasola A, Geuna M (2001) A flow cytometry assay simultaneously detects independent apoptotic parameters. Cytometry 45:151–157
Kramer N, Walzl A, Unger C, Rosner M, Krupitza G, Hengstschläger M, Dolznig H (2013) In vitro cell migration and invasion assays. Mutat Res 752:10–24
Richards RJ (2003) Responsibility for statistical analyses. Endocr Pract 9:329
Zhu R, Zou ST, Wan JM, Li W, Li XL, Zhu W (2013) BTG1 inhibits breast cancer cell growth through induction of cell cycle arrest and apoptosis. Oncol Rep 30:2137–2144
Manjili MH, Najarian K, Wang XY (2012) Signatures of tumor-immune interactions as biomarkers for breast cancer prognosis. Future Oncol 8:703–711
Martinez-Outschoorn UE, Pavlides S, Sotgia F, Lisanti MP (2011) Mitochondrial biogenesis drives tumor cell proliferation. Am J Pathol 178:1949–1952
Koff A, Cross F, Fisher A, Schumacher J, Leguellec K, Philippe M, Roberts JM (1991) Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell 66:1217–1228
Tirone F (2001) The gene PC3 (TIS21/BTG2), prototype member of the PC3/BTG/TOB family: regulator in control of cell growth, differentiation, and DNA repair? J Cell Physiol 187:155–165
Nicholson DW, Thornberry NA (2003) Apoptosis. Life and death decisions. Science 299:214–215
Corjay MH, Kearney MA, Munzer DA, Diamond SM, Stoltenborg JK (1998) Antiproliferative gene BTG1 is highly expressed in apoptotic cells in macrophage-rich areas of advanced lesions in Watanabe heritable hyperlipidemic rabbit and human. Lab Invest 78:847–858
Lee H, Cha S, Lee MS, Cho GJ, Choi WS, Suk K (2003) Role of antiproliferative B cell translocation gene-1 as an apoptotic sensitizer in activation-induced cell death of brain microglia. J Immunol 171:5802–5811
Nahta R, Yuan LX, Fiterman DJ, Zhang L, Symmans WF, Ueno NT, Esteva FJ (2006) B cell translocation gene 1 contributes to antisense Bcl-2-mediated apoptosis in breast cancer cells. Mol Cancer Ther 5:1593–1601
Wiseman BS, Werb Z (2002) Stromal effects on mammary gland development and breast cancer. Science 296:1046–1049
Alok C, Bharat B (2002) Nuclear factor-kappa Band cancer: its role in prevention and therapy. Biochem Phamacol 64:883–888
Virós D, Camacho M, Zarraonandia I, García J, Quer M, Vila L, León X (2013) Prognostic role of MMP-9 expression in head and neck carcinoma patients treated with radiotherapy or chemoradiotherapy. Oral Oncol 49:322–325
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, G.G., Wang, Y.D., Cheng, Y.J. et al. The expression of BTG1 is downregulated in nasopharyngeal carcinoma and possibly associated with tumour metastasis. Mol Biol Rep 41, 5979–5988 (2014). https://doi.org/10.1007/s11033-014-3475-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3475-0