Abstract
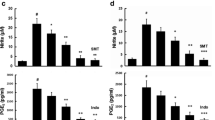
Grossamide, a representative lignanamide in hemp seed, has been reported to possess potential anti-inflammatory effects. However, the potential anti-neuroinflammatory effects and underlying mechanisms of action of grossamide are still unclear. Therefore, the present study investigated the possible effects and underlying mechanisms of grossamide against lipopolysaccharide (LPS)-induced inflammatory response in BV2 microglia cells. BV2 microglia cells were pre-treated with various concentrations of grossamide before being stimulated with LPS to induce inflammation. The levels of pro-inflammatory cytokines were determined using the enzyme-linked immunoassay (ELISA) and mRNA expression levels were measured by real-time PCR. The translocation of nuclear factor-kappa B (NF-κB) and contribution of TLR4-mediated NF-κB activation on inflammatory effects were evaluated by immunostaining and Western blot analysis. This study demonstrated that grossamide significantly inhibited the secretion of pro-inflammatory mediators such as interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α), and decreased the level of LPS-mediated IL-6 and TNF-α mRNA. In addition, it significantly reduced the phosphorylation levels of NF-κB subunit p65 in a concentration-dependent manner and suppressed translocation of NF-κB p65 into the nucleus. Furthermore, grossamide markedly attenuated the LPS-induced expression of Toll-like receptor 4 (TLR4) and myeloid differentiation factor 88 (MyD88). Taken together, these data suggest that grossamide could be a potential therapeutic candidate for inhibiting neuroinflammation in neurodegenerative diseases.





Similar content being viewed by others
References
Graeber MB, Li W, Rodriguez ML (2011) Role of microglia in CNS inflammation. FEBS Lett 585:3798–3805
Block ML, Hong JS (2005) Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 76:77–98
Smith JA, Das A, Ray SK, Banik NL (2012) Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull 87:10–20
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140:918–934
O’Neill LA, Kaltschmidt C (1997) NF-κB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci 20:252–258
Hayden MS, Ghosh S (2008) Shared principles in NF-kappaB signaling. Cell 132:344–362
Kaminska B, Mota M, Pizzi M (2016) Signal transduction and epigenetic mechanisms in the control of microglia activation during neuroinflammation. Biochim Biophys Acta 1862:339–351
Tan Y, Kagan JC (2014) A cross-disciplinary perspective on the innate immune responses to bacterial lipopolysaccharide. Mol Cell 54:212–223
Richard MN, Ganguly R, Steigerwald SN, Al-Khalifa A, Pierce GN (2007) Dietary hempseed reduces platelet aggregation. J Thromb Haemost 5:424–425
Lee MJ, Park SH, Han JH, Hong YK, Hwang S, Lee S, Kim D, Han SY, Kim ES, Cho KS (2011) The effects of hempseed meal intake and linoleic acid on Drosophila models of neurodegenerative diseases and hypercholesterolemia. Mol Cells 31:337–342
Hong S, Sowndhararajan K, Joo T, Lim C, Cho H, Kim S, Kim G, Jhoo J (2015) Ethanol and supercritical fluid extracts of hemp seed (Cannabis sativa L.) increase gene expression of antioxidant enzymes in HepG2 cells. Asian Pac J Reprod 4:147–152
Chen T, He J, Zhang J, Li X, Zhang H, Hao J, Li L (2012) The isolation and identification of two compounds with predominant radical scavenging activity in hempseed (seed of Cannabis sativa L.). Food Chem 134:1030–1037
Cao J, Li Z, Chen J, Li H (2005) Influence of semen cannabis iol on NO, SOD, GSH-Px, MDA in D-galactose-induced Aging Mice Serum. J Sichuan Tradit Chin Med 23(3):29–30
Wu SH, Guo YY, Zhang ST, Yang Y, Wang R, Zhang XY, Li HB (2015) The anti-aging effect of semen cannabis oil on the sex difference of Bombyx mori L. Pharmacol Clin Chin Mater Med 31:100–103
Zhang M, Shen Y, Zhu Z, Wang H (1999) Reseach on anti-inflammation, anti-analgesic and anti-thrombus function of semen cannabis. Res Pract Chin Med 13:13–15
Li G, Cao Y, Wu S, Zhang Z, Zhang Y, Yang Y, Li H (2015) Influence of semen cannabis iol on liqid levels, inflammmatory cytokines and anti-oxidant of aging model mice. Pharmacol Clin Chin Mater Med 31:109–111
Su J, He H, Shi M, Xiong X, Chen L (2011) Protective effect of semen cannabis iol on learning and memory impairment mice induced by D-galactose. Chin J Clin Pharmacol Ther 16:1332–1339
Luo J, Zheng T, Mo Z, Wei Q (2003) Effects of extracts of fructus cannabis on learning and memory capacity and related substances of senile mice induced by D-galactose. J Beijing Normal Univ (Nat Sci) 39(3):386–389
Flores-Sanchez IJ, Verpoorte R (2008) Secondary metabolism in cannabis. Phytochem Rev 7:615–639
Sakakibara I, Ikeya Y, Hayashi K, Okada M, Maruno M (1995) Three acyclic bis-phenylpropane lignanamides from fruits of Cannabis sativa. Phytochemistry 38:1003–1007
Sakakibara I, Katsuhara T, Ikeya Y, Hayashi K, Mitsuhashi H (1991) Cannabisin A, an arylnaphthalene lignanamide from fruits of Cannabis sativa. Phytochemistry 30:3013–3016
Harborne JB, Smith TA, Sakakibara I, Ikeya Y, Hayashi K, Mitsuhashi H (1992) Three phenyldihydronaphthalene lignanamides from fruits of Cannabis sativa. Phytochemistry 31:3219–3223
Yan X, Tang J, dos Santos Passos C, Nurisso A, Simoes-Pires CA, Ji M, Lou H, Fan P (2015) Characterization of Lignanamides from Hemp seed (Cannabis sativa L.) and their antioxidant and acetylcholinesterase inhibitory activities. J Agric Food Chem 63:10611–10619
Sun J, Gu YF, Su XQ, Li MM, Huo HX, Zhang J, Zeng KW, Zhang Q, Zhao YF, Li J, Tu PF (2014) Anti-inflammatory lignanamides from the roots of Solanum melongena L. Fitoterapia 98:110–116
Girgih AT, Alashi A, He R, Malomo S, Aluko RE (2014) Preventive and treatment effects of a hemp seed (Cannabis sativa L.) meal protein hydrolysate against high blood pressure in spontaneously hypertensive rats. Eur J Nutr 53:1237–1246
Liou CJ, Len WB, Wu SJ, Lin CF, Wu XL, Huang WC (2014) Casticin inhibits COX-2 and iNOS expression via suppression of NF-kappaB and MAPK signaling in lipopolysaccharide-stimulated mouse macrophages. J Ethnopharmacol 158:310–316
Wun ZY, Lin CF, Huang WC, Huang YL, Xu PY, Chang WT, Wu SJ, Liou CJ (2013) Anti-inflammatory effect of sophoraflavanone G isolated from Sophora flavescens in lipopolysaccharide-stimulated mouse macrophages. Food Chem Toxicol 62:255–261
Park HY, Han MH, Park C, Jin CY, Kim GY, Choi IW, Kim ND, Nam TJ, Kwon TK, Choi YH (2011) Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem Toxicol 49:1745–1752
Mancino A, Lawrence T (2010) Nuclear factor-kappaB and tumor-associated macrophages. Clin Cancer Res 16:784–789
Kim BW, Koppula S, Kumar H, Park JY, Kim IW, More SV, Kim IS, Han SD, Kim SK, Yoon SH, Choi DK (2015) alpha-Asarone attenuates microglia-mediated neuroinflammation by inhibiting NF kappa B activation and mitigates MPTP-induced behavioral deficits in a mouse model of Parkinson’s disease. Neuropharmacology 97:46–57
Li N, Zhang X, Dong H, Zhang S, Sun J, Qian Y (2016) Lithium ameliorates LPS-induced astrocytes activation partly via inhibition of Toll-Like Receptor 4 expression. Cell Physiol Biochem 38:714–725
Bindukumar B, Mahajan S, Reynolds J, Hu Z, Sykes D, Aalinkeel R, Schwartz S (2008) Genomic and proteomic analysis of the effects of cannabinoids on normal human astrocytes. Brain Res 1191:1–11
Cai P, Fu X, Deng AG, Zhan XJ, Cai GM, Li SX (2010) Anti-aging effect of hemp seed oil, protein and lignanamide of bama on old mice. Cent S Pharm 3:003
Wang X, Yang X, Tang C (2007) Nutritional assessment of Hemp (Cannabis sativa L.) proteins. Mod Food Sci Technol 7:002
Brenneisen R (2007) Chemistry and analysis of phytocannabinoids and other Cannabis constituents. In: Marijuana and the Cannabinoids, Springer, New York, pp 17–49
Chen H, Olatunji OJ, Zhou Y (2016) Anti-oxidative, anti-secretory and anti-inflammatory activities of the extract from the root bark of Lycium chinense (Cortex Lycii) against gastric ulcer in mice. J Nat Med 70:610–619
Girgih AT, Udenigwe CC, Aluko RE (2011) In vitro antioxidant properties of hemp seed (Cannabis sativa L.) protein hydrolysate fractions. J Am Oil Chem Soc 88:381–389
Sakakibara I, Ikeya Y, Hayashi K, Mitsuhashi H (1992) Three phenyldihydronaphthalene lignanamides from fruits of Cannabis sativa. Phytochemistry 31:3219–3223
Kim BW, More SV, Yun YS, Ko HM, Kwak JH, Lee H, Suk K, Kim IS, Choi DK (2016) A novel synthetic compound MCAP suppresses LPS-induced murine microglial activation in vitro via inhibiting NF-κB and p38 MAPK pathways. Acta Pharmacol Sin 37:334–343
Zhai XT, Zhang ZY, Jiang CH, Chen JQ, Ye JQ, Jia XB, Yang Y, Ni Q, Wang SX, Song J, Zhu FX (2016) Nauclea officinalis inhibits inflammation in LPS-mediated RAW 264.7 macrophages by suppressing the NF-kappaB signaling pathway. J Ethnopharmacol 183:159–165
Lee KC, Chang HH, Chung YH, Lee TY (2011) Andrographolide acts as an anti-inflammatory agent in LPS-stimulated RAW264.7 macrophages by inhibiting STAT3-mediated suppression of the NF-kappaB pathway. J Ethnopharmacol 135:678–684
Lee JW, Choi YJ, Kim SI, Lee SY, Kang SS, Kim NH, Kwon YS, Lee HJ, Chun WJ, Kim SS (2013) Betulinic acid inhibits LPS-induced MMP-9 expression by suppressing NF-κB activation in BV2 microglial cells. Biomol Ther (Seoul) 19:431–437
Markus RP, Cecon E, Pires-Lapa MA (2013) Immune-pineal axis: nuclear factor κB (NF-κB) mediates the shift in the melatonin source from pinealocytes to immune competent cells. Int J Mol Sci 14:10979–10997
Sung HC, Liang CJ, Lee CW, Yen FL, Hsiao CY, Wang SH, Jiang-Shieh YF, Tsai JS, Chen YL (2015) The protective effect of eupafolin against TNF-alpha-induced lung inflammation via the reduction of intercellular cell adhesion molecule-1 expression. J Ethnopharmacol 170:136–147
Cho KH, Kim DC, Yoon CS, Ko WM, Lee SJ, Sohn JH, Jang JH, Ahn JS, Kim YC, Oh H (2016) Anti-neuroinflammatory effects of citreohybridonol involving TLR4-MyD88-mediated inhibition of NF-κB and MAPK signaling pathways in lipopolysaccharide-stimulated BV2 cells. Neurochem Int 95:55–62
Jiang L, Xu F, He W, Chen L, Zhong H, Wu Y, Zeng S, Li L, Li M (2016) CD200Fc reduces TLR4-mediated inflammatory responses in LPS-induced rat primary microglial cells via inhibition of the NF-kappa B pathway. Inflamm Res 65:521–532
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81473323); Key R&D program in Shandong Province (No. 2015GSF119025) and the China-Australia Centre for Health Science Research (Grant No. 2015GJ04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Luo, Q., Yan, X., Bobrovskaya, L. et al. Anti-neuroinflammatory effects of grossamide from hemp seed via suppression of TLR-4-mediated NF-κB signaling pathways in lipopolysaccharide-stimulated BV2 microglia cells. Mol Cell Biochem 428, 129–137 (2017). https://doi.org/10.1007/s11010-016-2923-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2923-7