Abstract
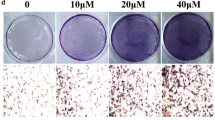
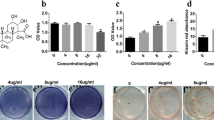
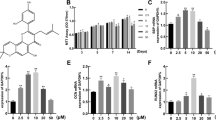
Many recent studies have suggested that bergapten (BP), a class of native compound with numerous biological activities such as anti-resorptive properties, may exert protective effects against postmenopausal bone loss. However, it remains unknown whether BP regulates or improves the osteogenic function of bone marrow stromal cells (BMSCs) in the treatment and prevention of osteoporosis. In our study, BMSCs were cultured in osteogenic induction medium with the addition of BP for 2 weeks and an ovariectomized mouse model of osteoporosis was used to investigate the anti-resorptive effect of BP by gavage administration for 3 months. The concentrations of BP used were 0.1, 1, and 10 μmol/L in vitro and the gavage dose was 20 mg/kg/d. The result of our study indicated that BP promotes the expression of alkaline phosphatase (ALP) by BMSCs in vitro in a dose-dependent manner, as revealed by ALP staining. Runt-related transcription factor 2 and osteocalcin were up-regulated both in vitro and vivo, while osterix and collagen Iα1, assessed by immunofluorescence and immunohistochemistry, were correspondingly raised in the presence of BP in BMSCs in vitro. In addition, a protective effect of BP against ovariectomy-induced bone loss was found by distal femur micro-CT scanning, with improvements of bone metabolism parameters such as bone mineral density, trabecular number, and trabecular separation. Furthermore, WNT/β-catenin signaling was activated in the presence of BP in BMSCs in osteogenic culture. Finally, BP promoted differentiation of BMSCs into osteoblasts by up-regulation of the WNT/β-catenin pathway.
Graphical abstract






Similar content being viewed by others
References
Lock CA, Lecouturier J, Mason JM, Dickinson HO (2006) Lifestyle interventions to prevent osteoporotic fractures: a systematic review. Osteoporos Int 17:20–28. doi:10.1007/s00198-005-1942-0
Kanis JA (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 4:368–381
Leung PC, Siu WS (2013) Herbal treatment for osteoporosis: a current review. J Tradit Complement Med 3:82–87. doi:10.4103/2225-4110.110407
Notelovitz M (1997) Estrogen therapy and osteoporosis: principles and practice. Am J Med Sci 313:2–12
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Writing Group for the Women’s Health Initiative I (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. JAMA 288:321–333
Owen M (1988) Marrow stromal stem cells. J Cell Sci Suppl 10:63–76
Nyandege AN, Slattum PW, Harpe SE (2015) Risk of fracture and the concomitant use of bisphosphonates with osteoporosis-inducing medications. Ann Pharmacother 49:437–447. doi:10.1177/1060028015569594
Pathak MA, Fitzpatrick TB (1992) The evolution of photochemotherapy with psoralens and UVA (PUVA): 2000 BC to 1992 AD. J Photochem Photobiol B 14:3–22
Panno ML, Giordano F, Rizza P, Pellegrino M, Zito D, Giordano C, Mauro L, Catalano S, Aquila S, Sisci D, De Amicis F, Vivacqua A, Fuqua SW, Ando S (2012) Bergapten induces ER depletion in breast cancer cells through SMAD4-mediated ubiquitination. Breast Cancer Res Treat 136:443–455. doi:10.1007/s10549-012-2282-3
Panno ML, Giordano F, Mastroianni F, Palma MG, Bartella V, Carpino A, Aquila S, Ando S (2010) Breast cancer cell survival signal is affected by bergapten combined with an ultraviolet irradiation. FEBS Lett 584:2321–2326. doi:10.1016/j.febslet.2010.04.001
Sumiyoshi M, Sakanaka M, Taniguchi M, Baba K, Kimura Y (2014) Anti-tumor effects of various furocoumarins isolated from the roots, seeds and fruits of Angelica and Cnidium species under ultraviolet a irradiation. J Nat Med 68:83–94. doi:10.1007/s11418-013-0774-z
Yu J, Wang L, Walzem RL, Miller EG, Pike LM, Patil BS (2005) Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J Agric Food Chem 53:2009–2014. doi:10.1021/jf0484632
Liu WX, Jia FL, He YY, Zhang BX (2012) Protective effects of 5-methoxypsoralen against acetaminophen-induced hepatotoxicity in mice. World J Gastroenterol 18:2197–2202. doi:10.3748/wjg.v18.i18.2197
Bose SK, Dewanjee S, Sahu R, Dey SP (2011) Effect of bergapten from heracleum nepalense root on production of proinflammatory cytokines. Nat Prod Res 25:1444–1449. doi:10.1080/14786410902800665
Zheng M, Ge Y, Li H, Yan M, Zhou J, Zhang Y (2014) Bergapten prevents lipopolysaccharide mediated osteoclast formation, bone resorption and osteoclast survival. Int Orthop 38:627–634. doi:10.1007/s00264-013-2184-y
Tang CH, Yang RS, Chien MY, Chen CC, Fu WM (2008) Enhancement of bone morphogenetic protein-2 expression and bone formation by coumarin derivatives via p38 and ERK-dependent pathway in osteoblasts. Eur J Pharmacol 579:40–49. doi:10.1016/j.ejphar.2007.10.013
Zhang Q, Qin L, He W, Van Puyvelde L, Maes D, Adams A, Zheng H, De Kimpe N (2007) Coumarins from Cnidium monnieri and their antiosteoporotic activity. Planta Med 73:13–19. doi:10.1055/s-2006-951724
Komori T (2015) Animal models for osteoporosis. Eur J Pharmacol. doi:10.1016/j.ejphar.2015.03.028
Kuznetsov S, Gehron Robey P (1996) Species differences in growth requirements for bone marrow stromal fibroblast colony formation in vitro. Calcif Tissue Int 59:265–270
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M (2001) Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2:165–171
Meunier P, Aaron J, Edouard C, Vignon G (1971) Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res 80:147–154
Lane NE, Kelman A (2003) A review of anabolic therapies for osteoporosis. Arthritis Res Ther 5:214–222. doi:10.1186/ar797
Bianco P, Kuznetsov SA, Riminucci M, Gehron Robey P (2006) Postnatal skeletal stem cells. Methods Enzymol 419:117–148. doi:10.1016/s0076-6879(06)19006-0
Riggs BL, Khosla S, Melton LJ 3rd (1998) A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res 13:763–773. doi:10.1359/jbmr.1998.13.5.763
Das S, Crockett JC (2013) Osteoporosis—a current view of pharmacological prevention and treatment. Drug Des Dev Ther 7:435–448. doi:10.2147/DDDT.S31504
Heiss C, Govindarajan P, Schlewitz G, Hemdan NY, Schliefke N, Alt V, Thormann U, Lips KS, Wenisch S, Langheinrich AC, Zahner D, Schnettler R (2012) Induction of osteoporosis with its influence on osteoporotic determinants and their interrelationships in rats by DEXA. Med Sci Monit 18:Br199–Br207
Cosman F (2014) Combination therapy for osteoporosis: a reappraisal. Bonekey Rep 3:518. doi:10.1038/bonekey.2014.13
Govindarajan P, Schlewitz G, Schliefke N, Weisweiler D, Alt V, Thormann U, Lips KS, Wenisch S, Langheinrich AC, Zahner D, Hemdan NY, Bocker W, Schnettler R, Heiss C (2013) Implications of combined ovariectomy/multi-deficiency diet on rat bone with age-related variation in bone parameters and bone loss at multiple skeletal sites by DEXA. Med Sci Monit Basic Res 19:76–86. doi:10.12659/MSMBR.883815
Gambacciani M, Levancini M (2015) Hormone replacement therapy: who should be treated? Minerva Ginecol 67:249–255
Maclennan AH (2009) Evidence-based review of therapies at the menopause. Int J Evid Based Healthc 7:112–123. doi:10.1111/j.1744-1609.2009.00133.x
Meng F, Xiong Z, Sun Y, Li F (2004) Coumarins from Cnidium monnieri (L.) and their proliferation stimulating activity on osteoblast-like UMR106 cells. Pharmazie 59:643–645
Lerner UH, Ohlsson C (2015) The WNT system: background and its role in bone. J Int Med. doi:10.1111/joim.12368
Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C (2005) Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 8:727–738. doi:10.1016/j.devcel.2005.02.013
Day TF, Guo X, Garrett-Beal L, Yang Y (2005) Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 8:739–750. doi:10.1016/j.devcel.2005.03.016
Glass DA 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8:751–764. doi:10.1016/j.devcel.2005.02.017
Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML, Deng L, Clemens TL, Williams BO (2005) Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem 280:21162–21168. doi:10.1074/jbc.M501900200
Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB, Feng JQ, Bonewald LF, Kneissel M (2010) Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol 30:3071–3085. doi:10.1128/MCB.01428-09
Hwang JH, Cha PH, Han G, Bach TT, Min DS, choi KY (2015) Euodia sutchuenensis dode extract stimulates osteoblast differentiation via Wnt/beta-catenin pathway activation. Exp Mol Med 47:e152. doi:10.1038/emm.2014.115
Lim JJ, Li X, Lu J, Pedego TM, Demer L, Tintut Y (2015) Protective role of Smad6 in inflammation-induced valvular cell calcification. J Cell Biochem. doi:10.1002/jcb.25186
Frey JL, Li Z, Ellis JM, Zhang Q, Farber CR, Aja S, Wolfgang MJ, Clemens TL, Riddle RC (2015) Wnt-Lrp5 signaling regulates fatty acid metabolism in the osteoblast. Mol Cell Biol. doi:10.1128/mcb.01343-14
Acknowledgments
We thank Zhang Xiao-dong (Department of Medical Image, The Third Affiliated Hospital of Southern Medical University, Guangdong, PR China) for excellent technical support.
Author contribution
Design of the study: X J-j, Z W-j, Z X-t and Z W-l. Acquisition of data: X J-j, Z W-j, Z X-t, Z W-l, W X-x, Y S-h and J F. Interpretation of data: W X-x, Y S-h, J F, Z Y-x and C F-n . Manuscript preparation: X J-j, Z W-j, Z X-t, Z W-l and L S-l.
Funding
This work was supported by Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethics approval
Ethical approval was given by the Medical Ethics Committee of Southern Medical University.
Provenance and peer review
Not commissioned; externally peer reviewed.
Additional information
Ji-jie Xiao, Wen-ji Zhao, Xin-tao Zhang, and Wen-long Zhao have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2015_2517_MOESM1_ESM.jpg
Supplementary Fig. 1. BP promotes the expression of RUNX2, OCN, β-catenin and GSK-3β. BMSCs were cultured in OIM alone without DMSO and BP or supplemented with BP (0.1 μM) for 2 weeks. BMSC lysates (20 μg protein) were prepared and subjected to western blot analysis using antibodies specific for OCN (a, b), RUNX2 (a, c), β-catenin (a, d) and GSK-3β (a, e) as described in the methods. The expression of OCN (b), RUNX2 (c), β-catenin (d) and GSK-3β (e) increased compared to the OIM group. Data are expressed as fold change versus OIM group. The results show that BP promotes the expression of RUNX2, OCN, β-catenin and GSK-3β compared to the OIM group. * P < 0.05 compared to OIM alone. Columns represent the mean ± SEM from nine wells per group (b–e). Below is the link to the electronic supplementary material. Supplementary material 1 (JPEG 192 kb)
11010_2015_2517_MOESM2_ESM.jpg
Supplementary Fig. 2. BP has no effect on the protein expression of RUNX2, OCN, β-catenin and GSK-3β in the absence of differentiation medium. BP (1–10 μM) did not alter the protein expression of OCN (a, b), RUNX2 (a, c), β-catenin (a, d) or GSK-3β (a, e) when cells were cultured for 14 days in the absence of differentiation medium. Columns represent the mean ± SEM from nine wells per group (b–e). Supplementary material 2 (JPEG 202 kb)
11010_2015_2517_MOESM3_ESM.jpg
Supplementary Fig. 3. BD did not have significant effect on endogenous stem cells as results of IHC. Through the characteristic markers of marrow stromal cells, such as CD105 (a-a’’ and c) and CD106 (b-b’’ and d), endogenous stem cells did not change significantly after BD treatment. Compared to OVX+BP group (a’’ and b’’), the expression intensity of CD105 and CD106 in OVX group (a’ and b’) and Sham group (a and b) got even distribution under microscope in IHC staining and had no significant difference between each other. n = 10. All values are shown as mean ± SEM of data from three independent experiments (c–d). Supplementary material 3 (JPEG 606 kb)
Rights and permissions
About this article
Cite this article
Xiao, Jj., Zhao, Wj., Zhang, Xt. et al. Bergapten promotes bone marrow stromal cell differentiation into osteoblasts in vitro and in vivo. Mol Cell Biochem 409, 113–122 (2015). https://doi.org/10.1007/s11010-015-2517-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2517-9