Abstract
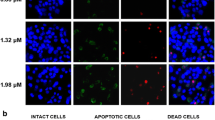
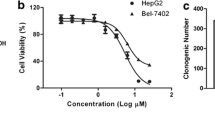
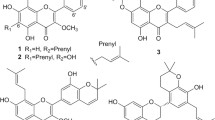
Calycopterin is a flavonoid compound isolated from Dracocephalum kotschyi that has multiple medical uses, as an antispasmodic, analgesic, anti-hyperlipidemic, and immunomodulatory agents. However, its biological activity and the mechanism of action are poorly investigated. Herein, we investigated the apoptotic effect of calycopterin against the human hepatoblastoma cancer cell (HepG2) line. We discovered that calycopterin-treated HepG2 cells were killed off by apoptosis in a dose-dependent manner within 24 h, and was characterized by the appearance of nuclear shrinkage, cleavage of poly (ADP-ribose) polymerase and DNA fragmentation. Calycopterin treatment also affected HepG2 cell viability: (a) by inhibiting cell cycle progression at the G2/M transition leading to growth arrest and apoptosis; (b) by decreasing the expression of mitotic kinase cdc2, mitotic phosphatase cdc25c, mitotic cyclin B1, and apoptotic factors pro-caspases-3 and -9; and (c) increasing the levels of mitochondrial apoptotic-related proteins, intracellular levels of reactive oxygen species, and nitric oxide. We further examined the phosphorylation of extracellular signal-related kinase (ERK 1/2), c-Jun N-terminal kinase, and p-38 mitogen-activated protein kinases (MAPKs) and found they all were significantly increased in HepG2 cells treated with calycopterin. Interestingly, we discovered that treated cells had significantly lower Akt phosphorylation. This mode of action for calycopterin in our study provides strong support that inhibition of PI3K/Akt and activation of MAPKs are pivotal in G2/M cell cycle arrest and apoptosis of human hepatocarcinoma cells mediated by calycopterin.











Similar content being viewed by others
References
Kasibhatla S, Tseng B (2003) Why target apoptosis in cancer treatment. Mol Cancer Ther 2:573–580
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Ghobrial IM, Witzig TE, Adjei AA (2005) Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin 55:178–194
Reed JC (2000) Mechanisms of apoptosis. Am J Pathol 157:1415–1430
Evan GI, Vousden KH (2001) Proliferation cell cycle and apoptosis in cancer. Nature 411:342–348
Circu ML, Aw TY (2001) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 48:749–762
Scorrano L, Korsmeyer SJ (2003) Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun 9:437–444
Lee KH (2010) Discovery and development of natural product-derived chemotherapeutic agents based on a medicinal chemistry approach. J Nat Prod 73:500–516
Karrer W, Venkataraman K (1935) Identity of calycopterin and thapsin. Nature 135:878
Ratnagiriswaran AN, Sehra KB, Venkataraman K (1934) The anthelminthic constituent of the leaves of Calycopteris floribunda. Biohem J 28:1964–1967
Feng X, Liang N, Zhu D, Gao Q, Peng L, Dong H, Yue Q, Liu H, Bao J, Zhang J, Hao J, Gao Y, Yu X, Sun J (2013) Resveratrol inhibits β-amyloid-induced neuronal apoptosis through regulation of SIRT1-ROCK1 signaling pathway. PLoS ONE 8:e9888
Zhang W, Wang X, Chen T (2012) Resveratrol induces apoptosis via a Bak-mediated intrinsic pathway in human lung adenocarcinoma cells. Cell Signal 24:1037–1046
Kiaei M, Kipiani K, Petri S, Chen J, Calingasan NY, Beal MF (2005) Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener Dis 2:246–254
Paris D, Ganey NJ, Laporte V, Patel NS, Beaulieu-Abdelahad D, Bachmeier CA, March a, Ait-Ghezala G, Mullan MJ (2010) Reduction of beta-amyloid pathology by celastrol in a transgenic mouse model o Alzheimer’s disease. J Neuroinflamm 8:7–17
Lofrumento DD, Nicolardi G, Cianciulli A, De Nuccio F, La Pesa V, Carofiglio V, Dragone T, Calvello R, Panaro MA (2014) Neuroprotective effects of resveratrol in an MPTP mouse model of Parkinson’s-like disease: possible role of SOCS-1 in reducing pro-inflammatory responses. Innate Immun 20:249–260
Sethi G, Ahn KS, Pandey MK, Aggarwal BB (2007) Celastrol a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB activation. Blood 109:2727–2735
Sha M, Ye J, Zhang LX, Luan ZY, Chen YB (2013) Celastrol induces apoptosis of gastric cancer cells by miR-146a inhibition of NF-κB activity. Cancer Cell Int 13:50–55
Yan FG, Wang M, Li JM, Cheng H, SuL J, Wang X, Wu H, Xia L, Li X, Chang HC, Li Q (2012) Gambogenic acid induced mitochondrial-dependent apoptosis and referred to Phospho-Erk1/2 and Phospho-p38 MAPK in human hepatoma HepG2 cells. Environ Toxicol Pharmacol 33:181–190
Mihara M, Moll UN (2013) Detection of mitochondrial localization of p 53. Methods Mol Biol 234:203–209
Farimani MM, Namazi Sarvestani N, Ansari N, Khodagholi F (2011) Calycopterin promotes survival and outgrowth of neuron-like PC12 cells by attenuation of oxidative- and ER-stress-induced apoptosis along with inflammatory response. Chem Res Toxicol 24:2280–2292
Sarvestani NN, Khodagholi F, Ansari N, Farimani MN (2013) Involvement of p-CREB and phase II detoxifying enzyme system in neuroprotection mediated by the flavonoid calycopterin isolated from Dracocephalumkotschyi. Phytomedicine 15:939–946
Lammer C, Wagerer S, Saffrich R, Mertens D, Ansorge W, Hoffmann I (1998) The cdc25B phosphatase is essential for the G2/M phase transition in human cells. J Cell Sci 111:2445–2453
Sgarlata C, Pérez-Martín J (2005) The cdc25 phosphatase is essential for the G2/M phase transition in the basidiomycete yeast Ustilago maydis. Mol Microbiol 58:1482–1496
Jiang X, Wang X (2004) Cytochrome C mediated apoptosis. Annu Rev Biochem 73:87–106
Goping IS, Gross A, Lavoie JN, Nguyen M, Jemmerson R, Roth K (1998) Regulated targeting of BAX to mitochondria. J Cell Biol 143:207–215
Shimizu S, Narita M, Tsujimoto Y (1999) Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 3:483–487
Kuwana T, Newmeyer DD (2003) Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol 15:691–699
Swamy SM, Huat BT (2003) Intracellular glutathione depletion and reactive oxygen species generation are important in alpha-hederin-induced apoptosis of P388 cells. Mol Cell Biochem 245:127–139
Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC (1998) Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318–1321
Kowaltowski AJ, Vercesi AE, Fiskum G (2000) Bcl-2 prevents mitochondrial permeability transition and cytochrome c release via maintenance of reduced pyridine nucleotides. Cell Death Differ 7:903–910
Atten MJ, Godoy-Romero E, Attar BM, Milson T, Zopel M, Holian O (2005) Resveratrol regulates cellular PKC alpha and delta to inhibit growth and induce apoptosis in gastric cancer cells. Invest New Drugs 23:111–119
Sampath D, Rao VA, Plunkett W (2003) Mechanisms of apoptosis induction by nucleoside analogs. Oncogene 22:9063–9074
Naowaratwattana W, De-Eknamkul W, De Mejia EG (2013) Phenolic-containing organic extracts of mulberry (Morus alba L.) leaves inhibit HepG2 hepatoma cells through G2/M phase arrest, induction of apoptosis, and inhibition of topoisomerase II alpha activity. J Med Food 13:1045–1056
Porter AG, Janicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6:99–104
Hussain AR, Al-Rasheed PS, Manogaran M, Al-Hussein KA, Platanias LC, Al Uddin S (2006) Curcumin induces apoptosis via inhibition of PI3’- kinase/AKT pathway in acute T cell leukemias. Apoptosis 11:245–254
Gururajan M, Dasu T, Shahidain S, Jennings CD, Robertson DA, Rangnekar VM, Bondada S (2007) Spleen tyrosine kinase (Syk), a novel target of curcumin, is required for B lymphoma growth. J Immunol 178:111–121
Johnson GL, Lapadat R (2002) Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911–1912
Hirose Y, Katayama M, Stokoe D, Haas-Kogan DA, Berger MS, Pieper RO (2003) The p38 mitogen-activated protein kinase pathway links the DNA mismatch repair system to the G2 checkpoint and to resistance to chemotherapeutic DNA-methylating agents. Mol Cell Biol 23:8306–8315
Chang L, Karin M (2001) Mammalian MAP kinase signaling cascades. Nature 410:37–40
Wei F, Xie Y, Tao L, Tang D (2010) Both ERK1 and ERK2 kinases promote G2/M arrest in etoposide-treated MCF7 cells by facilitating ATM activation. Cell Signal 22:1783–1789
Shaul YD, Seger R (2005) The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta 1773:1213–1226
Kumar S, Jiang MS, Adams JL, Lee JC (1999) Pyridinylimidazole compound SB 203580 inhibits the activity but not the activation of p38 mitogen-activated protein kinase. Biochem Biophys Res Commun 263:825–831
Acknowledgments
This study was supported by Shahid Beheshti University Research Council, and funds by the University of Arkansas for Medical Sciences and Center for Translational Neuroscience (UAMS-COBRE) to MK and grants from the National Center for Research Resources (5P20RR020146-09) and the National Institute of General Medical Sciences (8 P20 GM103425-09) as year-7 recruit of M. Kiaei. Authors greatly appreciated contribution of Dr. Kevin Phelan on this manuscript from valuable discussion, scientific input to proof reading.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esmaeili, M.A., Farimani, M.M. & Kiaei, M. Anticancer effect of calycopterin via PI3K/Akt and MAPK signaling pathways, ROS-mediated pathway and mitochondrial dysfunction in hepatoblastoma cancer (HepG2) cells. Mol Cell Biochem 397, 17–31 (2014). https://doi.org/10.1007/s11010-014-2166-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2166-4