Summary
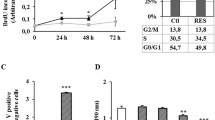
Resveratrol, a dietary phytoalexin, has emerged as a promising chemopreventive agent due to its antiproliferative and pro-apoptotic action toward cancer cells and its ability to inhibit tumor growth in animals. Gastric adenocarcinoma cells respond to resveratrol treatment with suppression of DNA synthesis, activation of nitric oxide synthase, induction of apoptosis and inhibition of total PKC and PKC α activity. Here we demonstrate that treatment of gastric adenocarcinoma SNU-1 cells with resveratrol results in time and concentration dependent accumulation of tumor suppressors p21(cip1/WAF -1) and p53 and is preceded by loss of membrane-associated PKC δ protein and a concomitant increase in cytosolic PKC α. Arrest of the cell cycle at transition of S to G2/M phases correlates with the profile of 3H-thymidine incorporation and accumulation of p21(cip1/WAF -1) and was temporally dependent on increase of p53. SNU-1 cells respond to resveratrol treatment with up-regulation of both Fas and Fas-L proteins, whereas in KATO-III cells, with deleted p53, only Fas-L is increased after resveratrol treatment. Although Fas and Fas-L proteins in SNU-1 cells and Fas-L in KATO-III cells were elevated within 24 h of cell treatment with low concentrations of resveratrol, significant apoptotic response at these concentrations was observed only after 48 h. Altogether, our findings indicate that resveratrol engages PKC α and δ signals in gastric adenocarcinoma SNU-1 cells prior to up-regulation of antiproliferative and pro-apoptotic signals. The specific cell death signals engaged by resveratrol appear to be cell type dependent and suggest that resveratrol has chemopreventive potential even after mutational changes have occurred.
Similar content being viewed by others
References
Jang M, Cai L, Udeani Go, Slowing KV, Thomas CF, Beecher CWW, Fong HHS, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM: Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275: 218–220, 1997
Mgbonyebi O, Russo J, Russo IH: Antiproliferative effect of synthetic resveratrol on human breast epithelial cells. Intern J Oncol 12: 865–869, 1998
Kawada N, Seki S, Inoue M, Kuroki T: Effect of antioxidants, resveratrol, quercetin, and N-acetylcysteine, on the functions of cultured rat hepatic stellate cells and Kupffer cell. Hepatol 27: 1265–1274, 1998
Lu R, Serrero G: Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. J Cellular Physiol 179: 297–304, 1999
Ragione DF, Cucciolla V, Borriello A, Pietra VD, Racioppi L, Soldati G, Manna C, Galletti P, Zappia V: Resveratrol arrests the cell division cycle at S/G2 phase transition. Biochem Biophys Res Commun 250: 53–58, 1998
Gautam SC, Xu YX, Dumaguin M, Janakiraman N, Chapman RA: Resveratrol selectively inhibits leukemia cells: A prospective agent for ex vivo bone marrow purging. Bone Marrow Transplant 25: 639–645, 2000
Wolter F, Akoglu B, Clausnitzer A, Stein J: Downregulation of the cyclin D1/Cdk4 complex occurs during resveratrol-induced cell cycle arrest in colon cancer cell lines. J Nutr 131: 2197–2203, 2001
Brakenhielm E, Cao R, Cao Y: Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes. FASEB J 15: 1798–1800, 2001
Kimura Y, Okuda H: Effects of naturally occurring stilbene glucosides from medicinal plants and wine, on tumour growth and lung metastasis in Lewis lung carcinoma-bearing mice. J Phar Pharmacol 52: 1287–1295, 2000
Mahady GB, Pendland SL: Resveratrol inhibits the growth of Helicobacter pylori in vitro. Am J Gastroenterol 95: 1849, 2000
Daroch F, Hoeneisen M, Gonzalez CL, Kawaguchi F, Salgado F, Solar H, Garcia A: In vitro antibacterial activity of Chilean red wines against Helicobacter pylori. Microbios 104: 79–85, 2001
Bernhard D, Tinhofer I, Tonko M, Hubl H, Ausserlechner MJ, Greil R, Kofler R, Csordas A: Resveratrol causes arrest in the S-phase prior to Fas-independent apoptosis in CEM-C7H2 acute leukemia cells. Cell Death Differ 7: 834–842, 2000
Park JW, Choi YJ, Jang MA, Lee YS, Jun DY, Suh SI, Baek WK, Suh MH, Jin IN, Kwon TK: Chemopreventive agent resveratrol, a natural product derived from grapes, reversibly inhibits progression through S and G2 phases of the cell cycle in U937 cells. Cancer Lett 163: 43–49, 2001
Joe AK, Liu H, Suzui M, Vural ME, Xiao D, Weinstein IB: Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin Cancer Res. 8: 88893–88903, 2002
Tessitore L, Davit A, Sarotto I, Caderni G: Resveratrol depresses the growth of colorectal aberrant crypt foci by affecting bax and p21CIP expression. Carcinogenesis 21: 1619–1622, 2000
Ahmad N, Adhami VM, Afaq F, Feyes DK, Mukhtar H: Resveratrol causes WAF-1/p21-mediated G1-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A432 cells. Clin Cancer Res 7: 1466–1473, 2001
Huang J, Gan J: Relationship between expression of tumor suppressor protein p21 and p53 and cell proliferation in gastric carcinoma. Hunan Yi Ke Da Xue Xue Bao 23: 441–443, 1998
Cho JH, Kim WH: Altered topographic expression of p21 WAF1/CIP1/SDI1, bcl2 and p53 during gastric carcinogenesis. Pathol Res Pract 194: 309–317, 1998
Xiangming C, Hokita A, Natsugoe S, Tanabe G, Baba M, Takao S, Kuroshima K, Aidou T: P21 expression is a prognostic factor in patients with p53-negative gastric cancer. Cancer Lett 148: 181–188, 2000
Kuwajerwala N, Cifuentes E, Gautam S, Menon M, Barrack ER, Reddy GP: Resveratrol induces prostate cancer cell entry into S phase and inhibits DNA synthesis. Cancer Res 62: 2488–2492, 2002
Thakkar K, Geahlen RL, Cushman M: Synthesis and protein-tyrosine kinase inhibitory activity of polyhydroxylated stilbene analogues of piceatannol. J Med Chem 36: 2950–2955, 1993
Stewart JR, Christman KL, O’Brian CA: Effects of resveratrol on the autophosphorylation of phorbol ester-responsive protein kinases. Inhibition of protein kinase D but not protein kinase C isozyme autophosphorylation. Biochem Pharmacol 60: 1355–1359, 2000
Stewart JR, Ward NE, Ioannides CG, O’Brian CA: Resveratrol preferentially inhibits protein kinase C-catalyzed phosphorylation of a cofactor-independent, arginine-rich protein substrate by a novel mechanism. Biochem 38: 13244–13251, 1999
Slater SJ, Seiz JL, Cook AC, Stagliano BA, Buzas CJ: Inhibition of protein kinase C by resveratrol. Biochim Biophys Acta 1637: 59–69, 2003
Heider UG, Sorescu D, Griendling KK, Vollmar AM, Dirsch VM: Resveratrol suppresses angiotensin II-induced Akt/protein kinase B and p70 S6 kinase phosphorylation and subsequent hypertrophy in rat aortic smooth muscle cells. Mol Pharmacol 62: 772–777, 2002
Holmes-McNary M, Baldwin AS Jr: Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB-kinase. Cancer Res 60: 4377–4383, 2000
Wolter F, Akoglu B, Clausnitzer A, Stein J: Downregulation of the cyclin D1/Cdk4 complex occurs during resveratrol-induced cell cycle arrest in colon cancer cell lines. J Nutr 131: 2197–2203, 2001.
Sawai H, Okazaki T, Takeda Y, Tashima M, Sawada H, Okuma M, Kishi S, Umehara H, Domae N: Ceremide-induced translocation of protein kinase C-delta and –epsilon to the cytosol: Implications in apoptosis. J Biol Chem 272: 2452–2458, 1997
Ghayur T, Hugunin M, Talanian RV, Ratnofsky S, Quinlan C, Emoto Y, Pandey P, Datta R, Huang Y, Kharbanda S, Allen H, Kamen R, Wong W, Kufe D: Proteolytic activation of protein kinase C delta by an ICE/CED 3-like protease induces characteristics of apoptosis. J Exp Med 184: 2399–2404, 1996
Cross T, Griffiths G, Deacon E, Sallis R, Gough M, Watters D, Lord JM: PKC-delta is an apoptotic lamin kinase. Oncogene 19: 2331–2337, 2000.
Fujii T, Garcia-Bermejo ML, Bernabo JL, Caamano J, Ohba M, Kuroki T, Li L, Yuspa SH, Kazanietz MG: Involvement of protein kinase C delta (PKC delta) in phorbol ester-induced apoptosis in LNCaP prostate cancer cells. Lack of proteolytic cleavage of PKC delta. J Biol Chem 275: 7574–7582, 2000
Atten MJ, Attar M, Milson T, Holian O: Resveratrol-induced inactivation of human gastric adenocarcinoma cells through a protein kinase C-mediated mechanism. Biochem Pharmacol 62: 1423–1432, 2001
Holian O, Wahid S, Atten MJ, Attar BM: Inhibition of gastric cancer cell proliferation by resveratrol: Role of nitric oxide. Am J Physiol Gastriontest Liver Physiol 282: G809–G816, 2002
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ: Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951
Gescher A: Analogs of staurosporine: Potential anticancer drugs? Gen Pharm 31: 721–728, 1998
Hofmann J: Modulation of protein kinase C in antitumor treatment. Rev Physiol Biochem Pharmacol 142–196, 2001
Blobe GC, Hannun YA: Regulation of protein kinase C and role in cancer biology. Cancer Met Rev 13: 411–431, 1994
Musashi M, Ota S, Shiroshita N: The role of protein kinase C isoforms in cell proliferation and apoptosis. Int J Hematol 72: 12–19, 2000
Blobe GC, Stribling S, Obeid LM, Hannun YA: Protein kinase C isoenzymes: Regulation and function. Cancer Sur 27: 213–248, 1996
Cross T, Griffiths G, Deacon E, Sallis R, Gough M, Watters D, Lord JM: PKC-delta is an apoptotic lamin kinase. Oncogene 19: 2331–2337, 2000
Ghayur T, Hugunin M, Talanian RV, Ratnofsky S, Quinlan C, Emoto Y, Pandey P, Datta R, Huang Y, Kharbanda S, Allen H, Kamen R, Wong W, Kufe D: Proteolytic activation of protein kinase C delta by an ICE/CED 3-like protease induces characteristics of apoptosis. J Exp Med 184: 2399–2404, 1996
Visnjic D, Batanic D, Banfic H: Different role of protein kinase C alpha and delta isoforms in the regulation of neuronal sphingomyelinase activity in HL-60 cells. Biochem J 344: 921–928, 1999
Rybin VO, Steinberg SF: Protein kinase C isoform expression and regulation in the developing rat heart. Circ Res 74: 299–309, 1994
Assert RH, Schatz H, Pfeiffer: Upregulation of PKC delta- and downregulation of alpha-mRNA and protein by phorbol ester in human T84 cells. FEBS Letters 388: 195–199, 1996
Balasubramanian N, Advani SH, and Zingde SM: Protein kinase C isoforms in normal and chronic myeloid leukemic neutrophils. Distinct signal for PKC alpha by immunodetection on PVDF membrane, decreased expression of PKC alpha and increased expression of PKC delta in leukemic neutrophils. Leuk Res 22: 597–604, 1998
Palmieri L, Mameli M, Ronca G: Effect of resveratrol and some other natural compounds on tyrosine kinase activity and on cytolysis. Drugs Exp Clin Res 25: 79–85, 1999
El-Deiry WS: Regulation pf p53 downstream genes. Sem Cancer Biol 8: 345–357, 1998
Muller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M, Friedman SL, Galle PR, Stremmel W, Oren M, Krammer PH: p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J Exp Med 188: 2033–2045, 1998
Tsan MF, White JE, Maheshwari JG, Bremmer TA, Sacco J: Resveratrol induces Fas signalling-independent apoptosis in THP-1 human monocytic leukemia cells. Br J Haematol 109: 405–412, 2000
Delmas D, Rebe C, Lacour S, Filomenko R, Athias A,Gambert P, Chrkaoui-Malki M, Jannin B, Dubrez-Daloz L, Latruffe N, Losary E: Resveratrol-induced apoptosis is associated with Fas redistribution in the rafts and the formation of a death inducing signaling complex in colon cancer cells. J Biol Chem 278: 41482–41490, 2003
Mahyar-Roemer M, Katsen A, Mestres P, Roemer K: Resveratrol induces colon tumor cell apoptosis independently of p53 and preceded by epithelial differentiation, mitochondrial proliferation and membrane potential collapse. Int J Cancer 94: 615–622, 2001
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atten, M.J., Godoy-Romero, E., Attar, B.M. et al. Resveratrol regulates cellular PKC α and δ to inhibit growth and induce apoptosis in gastric cancer cells. Invest New Drugs 23, 111–119 (2005). https://doi.org/10.1007/s10637-005-5855-8
Issue Date:
DOI: https://doi.org/10.1007/s10637-005-5855-8