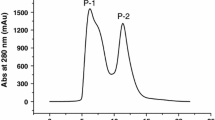
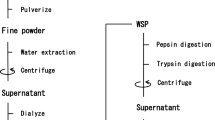
Abstract
Bioactive peptides are defined as protein-based components having nutritional value and have proved roles important for the human health. In this study inhibition of angiotensin converting enzyme (ACE) by protein-based hydrolysate extracted from walnut (Juglanse regia. L.) seeds was evaluated. The peptide fraction obtained by enzymatic hydrolysis with trypsin showed higher ACE-inhibitory and lower IC50 value (0.39 ± 0.05 mg/mL) than obtained by hydrolysis with chymotrypsin and proteinase K. The study of kinetics showed that by increasing the concentration of the trypsin hydrolysate from 0.01–0.5 mg/mL, Km increased, while Vmax decreased. Also the value of Ki was found to be 0.17 ± 0.01 mg/mL, which means that binding affinity for the substrate decreased in the presence of inhibitor. The structural studies of ACE demonstrated that, in comparison with a commercial antihypertension drug (enalapril), the trypsin hydrolysate had no effect on secondary structure and less tertiary structure changes of protein was observed.







Similar content being viewed by others
Abbreviations
- ACE:
-
Angiotensin converting enzyme
- FAPGG:
-
N-(3-[2-furyl]acryloyl)-l-phenylalanylglycylglycine
References
Albery WJ, Knowles JR (1976) Evolution of enzyme function and the development of catalytic efficiency. BioChemistry 15:5631–5640
Atlas SA (2007) The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm 13:9–20
Bhaskar N, Benila T, Radha C, Lalitha RG (2008) Optimization of enzymatic hydrolysis of visceral waste proteins of Catla (Catla catla) for preparing protein hydrolysate using a commercial protease. Bioresour Technol 99:335–343
Bolognesi B et al (2010) ANS binding reveals common features of cytotoxic amyloid species. ACS Chem Biol 5:735–740
Boschin G, Scigliuolo GM, Resta D, Arnoldi A (2014) ACE-inhibitory activity of enzymatic protein hydrolysates from lupin and other legumes. Food Chem 145:34–40
Brown NJ, Vaughan DE (1998) Angiotensin-converting enzyme inhibitors. Circulation 97:1411–1420
Campioni S et al (2010) A causative link between the structure of aberrant protein oligomers and their toxicity. Nat Chem Biol 6:140–147
Cao W, Zhang C, Ji H, Hao J (2012) Optimization of peptic hydrolysis parameters for the production of angiotensin I-converting enzyme inhibitory hydrolysate from Acetes chinensis through Plackett–Burman and response surface methodological approaches. J Sci Food Agric 92:42–48
Chen H-M, Muramoto K, Yamauchi F, Fujimoto K, Nokihara K (1998) Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J Agric Food Chem 46:49–53
Cragg GM, Newman DJ (2013) Natural products: a continuing source of novel drug leads. Biochim Biophys Acta 1830:3670–3695
Cushman D, Cheung H (1971) Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol 20:1637–1648
Daban J-R, Samsó M, Bartolomé S (1991) Use of Nile red as a fluorescent probe for the study of the hydrophobic properties of protein-sodium dodecyl sulfate complexes in solution. Anal Biochem 199:162–168
Fernández-Agulló A, Pereira E, Freire M, Valentão P, Andrade P, González-Álvarez J, Pereira J (2013) Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind Crops Prod 42:126–132
Fuglsang A, Nilsson D, Nyborg NC (2003) Characterization of new milk-derived inhibitors of angiotensin converting enzyme in vitro and in vivo. J Enzyme Inhib Med Chem 18:407–412
Girgih AT, Udenigwe CC, Li H, Adebiyi AP, Aluko RE (2011) Kinetics of enzyme inhibition and antihypertensive effects of hemp seed (Cannabis sativa L.) protein hydrolysates. J Am Oil Chem Soc 88:1767–1774
Hedstrom L (2002) Serine protease mechanism and specificity. Chem Rev 102:4501–4524
Hodgson JM, Croft KD (2006) Dietary flavonoids: effects on endothelial function and blood pressure. J Sci Food Agric 86:2492–2498
Huang W-Y, Davidge ST, Wu J (2013) Bioactive natural constituents from food sources—potential use in hypertension prevention and treatment. Crit Rev Food Sci Nutr 53:615–630
Iwaniak A, Minkiewicz P, Darewicz M (2014) Food-originating ACE inhibitors, including antihypertensive peptides, as preventive food components in blood pressure reduction. Compr Rev Food Sci Food Saf 13:114–134
Jamdar S, Rajalakshmi V, Pednekar M, Juan F, Yardi V, Sharma A (2010) Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chem 121:178–184
Khaket TP, Singh J (2016) Potential of plant’s dipeptidyl peptidase I & II homologs in generation of ace inhibitory peptides. Int J Pept Res Ther 23:81–90
Kraut J (1977) Serine proteases: structure and mechanism of catalysis. Annu Rev Biochem 46:331–358
Labuckas DO, Maestri DM, Perello M, Martínez ML, Lamarque AL (2008) Phenolics from walnut (Juglans regia L.) kernels: antioxidant activity and interactions with proteins. Food Chem 107:607–612
Mäkinen S, Johannson T, Gerd EV, Pihlava JM, Pihlanto A (2012) Angiotensin I-converting enzyme inhibitory and antioxidant properties of rapeseed hydrolysates. J Funct Foods 4:575–583
Newman DJ, Cragg GM, Snader KM (2003) Natural products as sources of new drugs over the period 1981–2002. J Nat Prod 66:1022–1037
Nilsang S, Lertsiri S, Suphantharika M, Assavanig A (2005) Optimization of enzymatic hydrolysis of fish soluble concentrate by commercial proteases. J Food Eng 70:571–578
Onuh JO, Girgih AT, Malomo SA, Aluko RE, Aliani M (2015) Kinetics of in vitro renin and angiotensin converting enzyme inhibition by chicken skin protein hydrolysates and their blood pressure lowering effects in spontaneously hypertensive rats. J Funct Foods 14:133–143
Pérez-Jiménez J, Arranz S, Tabernero M, Díaz-Rubio ME, Serrano J, Goñi I, Saura-Calixto F (2008) Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: extraction, measurement and expression of results. Food Res Int 41:274–285
Sackett DL, Wolff J (1987) Nile red as a polarity-sensitive fluorescent probe of hydrophobic protein surfaces. Anal Biochem 167:228–234
Sarmadi BH, Ismail A (2010) Antioxidative peptides from food proteins: a review. Peptides 31:1949–1956
Shalaby SM, Zakora M, Otte J (2006) Performance of two commonly used angiotensin-converting enzyme inhibition assays using FA-PGG and HHL as substrates. J Dairy Res 73:178–186
Shimakage A, Shinbo M, Yamada S (2012) ACE inhibitory substances derived from soy foods. J Biol Macromol 12:72–80
Sornwatana T, Bangphoomi K, Roytrakul S, Wetprasit N, Choowongkomon K, Ratanapo S (2015) Chebulin: Terminalia chebula Retz. fruit-derived peptide with angiotensin-I–converting enzyme inhibitory activity. Biotechnol Appl Biochem 62:746–753
Sze-Tao KWC, Sathe SK (2000) Walnuts (Juglans regia L): proximate composition, protein solubility, protein amino acid composition and protein in vitro digestibility. J Sci Food Agric 80:1393–1401
Tomatsu M, Shimakage A, Shinbo M, Yamada S, Takahashi S (2013) Novel angiotensin I-converting enzyme inhibitory peptides derived from soya milk. Food Chem 136:612–616
Vallabha VS, Tiku PK (2014) Antihypertensive peptides derived from soy protein by fermentation. Int J Pept Res Ther 20:161–168
Vermeirssen V, Van Camp J, Verstraete W (2002) Optimisation and validation of an angiotensin-converting enzyme inhibition assay for the screening of bioactive peptides. J Biochem Biophys Methods 51:75–87
Yodjun M, Karnchanatat A, Sangvanich P (2012) Angiotensin I-converting enzyme inhibitory proteins and peptides from the rhizomes of Zingiberaceae plants. Appl Biochem Biotechnol 166:2037–2050
Acknowledgements
The support of University of Tehran, International Scientific Studies & Collaboration (CISSC)-Ministry of Science, Research and Technology in Iran, Center of Excellence in Biothermodynamics (CEBiotherm), Center of Excellence for Walnut Improvement and Technology of Iran, Iran National Science Foundation (INSF) and Iran National Elites Foundation (INEF) and UNESCO Chair on Interdisciplinary Research in Diabetes, Iran Society of Biophysical Chemistry is gratefully acknowledged. The authors also acknowledge the Headquarter of Science and Technology Development in Medicinal Plants of Iran’s Vice-President for Science and Technology Affairs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All author declares that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Jahanbani, R., Ghaffari, M., Vahdati, K. et al. Kinetics Study of Protein Hydrolysis and Inhibition of Angiotensin Converting Enzyme by Peptides Hydrolysate Extracted from Walnut. Int J Pept Res Ther 24, 77–85 (2018). https://doi.org/10.1007/s10989-017-9594-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-017-9594-4