Abstract
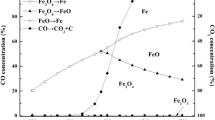
Low-grade hydrogen-containing gases can be converted into high-value pure hydrogen by chemical-looping hydrogen generation. Iron-based oxygen carrier is believed to be the most suitable oxygen carrier for CLHG. It is essential to investigate the reduction mechanism and kinetics of Fe2O3 with hydrogen. The reduction of Fe2O3 by H2 was conducted in a thermogravimetric analyzer at 973–1172 K. The phase analysis of reduction products in different reduction stages illustrated that Fe2O3 → Fe3O4 and Fe3O4 → FeO proceed simultaneously; hence, the reduction process is a two-step reaction, in the sequence of Fe2O3 → FeO and FeO → Fe. To investigate the reaction mechanisms for the two steps, the Hancock–Sharp method and the nonlinear fitting approach were applied to select the kinetic models. The reaction mechanisms of Fe2O3 → FeO can be described by nucleation and growth model. The activation energy is 36.74 ± 1.09 kJ mol−1, and reaction rate equation derived from Arrhenius law is estimated for Fe2O3 → FeO. As for FeO → Fe, the first stage is controlled by the phase-boundary reaction, and the oxygen diffusion affects the last stage of the reduction process.









Similar content being viewed by others
References
Crompton P, Wu Y. Energy consumption in China: past trends and future directions. Energy Econ. 2005;27(1):195–208.
Yi WJ, Zou LL, Jie G, Kai W, Wei YM. How can China reach its CO2 intensity reduction targets by 2020? a regional allocation based on equity and development. Energy Policy. 2011;39(5):2407–15.
Ben H, Mu W, Deng Y, Ragauskas AJ. Production of renewable gasoline from aqueous phase hydrogenation of lignin pyrolysis oil. Fuel. 2013;103:1148–53.
Eberle U, Müller B, von Helmolt R. Fuel cell electric vehicles and hydrogen infrastructure: status 2012. Energy Environ Sci. 2012;5(10):8780–98.
Zhang F, Zhao P, Niu M, Maddy J. The survey of key technologies in hydrogen energy storage. Int J Hydrog Energy. 2016;41(33):14535–52.
Ishida M, Jin H. A novel chemical-looping combustor without NOx formation. Ind Eng Chem Res. 1996;35(7):2469–72.
Hua X, Wang W. Chemical looping combustion: a new low-dioxin energy conversion technology. J Environ Sci. 2015;32:135–45.
Iliuta I, Tahoces R, Patience GS, Rifflart S, Luck F. Chemical-looping combustion process: kinetics and mathematical modeling. AIChE J. 2010;56(4):1063–79.
Zhou Z, Han L, Bollas GM. Model-based analysis of bench-scale fixed-bed units for chemical-looping combustion. Chem Eng J. 2013;233(11):331–48.
Zhou Z, Han L, Nordness O, Bollas GM. Continuous regime of chemical-looping combustion (CLC) and chemical-looping with oxygen uncoupling (CLOU) reactivity of CuO oxygen carriers. Appl Catal B. 2015;166:132–44.
Forero C, Gayán P, De Diego L, Abad A, García-Labiano F, Adánez J. Syngas combustion in a 500 W th chemical-looping combustion system using an impregnated Cu-based oxygen carrier. Fuel Process Technol. 2009;90(12):1471–9.
Inguanzo M, Domínguez A, Menéndez JA, Blanco CG, Pis JJ. On the pyrolysis of sewage sludge: the influence of pyrolysis conditions on solid, liquid and gas fractions. J Anal Appl Pyrolysis. 2002;63(1):209–22.
Remón J, Broust F, Valette J, Chhiti Y, Alava I, Fernandez-Akarregi AR, et al. Production of a hydrogen-rich gas from fast pyrolysis bio-oils: comparison between homogeneous and catalytic steam reforming routes. Int J Hydrog Energy. 2014;39(1):171–82.
Bridgwater AV. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy. 2012;38:68–94.
Abad A, Adánez J, Cuadrat A, García-Labiano F, Gayán P, Luis F. Kinetics of redox reactions of ilmenite for chemical-looping combustion. Chem Eng Sci. 2011;66(4):689–702.
Zhao H-Y, Cao Y, Orndorff W, Pan W-P. Study on modification of Cu-based oxygen carrier for chemical looping combustion. J Therm Anal Calorim. 2013;113(3):1123–8.
Leion H, Jerndal E, Steenari B-M, Hermansson S, Israelsson M, Jansson E, et al. Solid fuels in chemical-looping combustion using oxide scale and unprocessed iron ore as oxygen carriers. Fuel. 2009;88(10):1945–54.
Luo M, Wang S, Wang L, Lv M. Reduction kinetics of iron-based oxygen carriers using methane for chemical-looping combustion. J Power Sources. 2014;270(4):434–40.
Hua X, Wang W, Wang F. Performance and kinetics of iron-based oxygen carriers reduced by carbon monoxide for chemical looping combustion. Front Environ Sci Eng. 2015;9(6):1130–8.
Monazam ER, Breault RW, Siriwardane R. Reduction of hematite (Fe2O3) to wüstite (FeO) by carbon monoxide (CO) for chemical looping combustion. Chem Eng J. 2014;242(242):204–10.
Piotrowski K, Mondal K, Lorethova H, Stonawski L, Szymański T, Wiltowski T. Effect of gas composition on the kinetics of iron oxide reduction in a hydrogen production process. Int J Hydrog Energy. 2005;30(15):1543–54.
Lin HY, Chen YW, Li C. The mechanism of reduction of iron oxide by hydrogen. Thermochim Acta. 2003;400(1–2):61–7.
Sastri M, Viswanath R, Viswanathan B. Studies on the reduction of iron oxide with hydrogen. Int J Hydrog Energy. 1982;7(12):951–5.
Moon I-J, Rhee C-H, Min D-J. Reduction of hematite compacts by H2–CO gas mixtures. Steel Res. 1998;69(8):302–6.
Zhou A, Suzuki K, Sahajwalla V, Cadogan JM. A Mossbauer study of the gas-based direct reduction of iron ore fines and application of the Johnson–Mehl–Avrami kinetic model to the reduction process. Scand J Metall. 1999;28(2):65–9.
Bonalde A, Henriquez A, Manrique M. Kinetic analysis of the iron oxide reduction using hydrogen–carbon monoxide mixtures as reducing agent. ISIJ Int. 2005;45(9):1255–60.
Pineau A, Kanari N, Gaballah I. Kinetics of reduction of iron oxides by H2: part I: low temperature reduction of hematite. Thermochim Acta. 2006;447(1):89–100.
Slagtern Å, Swaan HM, Olsbye U, Dahl IM, Mirodatos C. Catalytic partial oxidation of methane over Ni-, Co- and Fe-based catalysts. Catal Today. 1998;46(2):107–15.
Perrenot B, Widmann G. TG and DSC kinetics of thermal decomposition and crystallization processes. J Therm Anal Calorim. 1991;37(8):1785–92.
Wiltowski T, Hinckley CC, Smith GV, Nishizawa T, Saporoschenko M, Shiley RH, et al. Kinetics and mechanisms of iron sulfide reductions in hydrogen and in carbon monoxide. J Solid State Chem. 1987;71(1):95–102.
Zhou Z, Han L, Bollas GM. Kinetics of NiO reduction by H2 and Ni oxidation at conditions relevant to chemical-looping combustion and reforming. Int J Hydrog Energy. 2014;39(16):8535–56.
Monazam ER, Breault RW, Siriwardane R. Kinetics of hematite to wüstite by hydrogen for chemical looping combustion. Energy Fuels. 2014;28(8):5406–14.
Acknowledgements
This work was supported by the National Key Technology R&D Program of China (2015BAD21B05 and 2014BAC24B00) and the National Natural Science Foundation of China (Grant No. 21477061). The authors also wish to express thanks to the Beijing Engineering Research Center of Biogas Centralized Utilization (Tsinghua University) for support of the experimental unit construction.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, X., Hua, X. et al. The reduction mechanism and kinetics of Fe2O3 by hydrogen for chemical-looping hydrogen generation. J Therm Anal Calorim 129, 1831–1838 (2017). https://doi.org/10.1007/s10973-017-6267-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6267-7