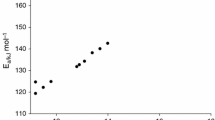
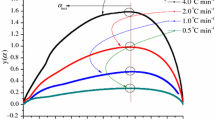
Abstract
Thermal decomposition of 20 mass% aqueous tert-butyl hydroperoxide (TBHP) is performed by confined apparatus as a model reaction. Inductive effects are verified by of hydroxyl and tert-butoxy radicals produced by the thermolysis of H2O2 and di-tert-butyl peroxide (DTBP), respectively. Solvent effects are performed by 20 mass% TBHP in acetone, n-butyl alcohol, decane, nonane, Nujol, toluene and H2O. Thermodynamic and kinetic features, such as exothermic onset temperature, adiabatic temperature rise, maximum self-heat rate, maximum pressure and maximum temperature, are thus measured and utilized to characterize these mentioned influences. Hydroxyl radical is firstly discovered to possess the highest inductive effect on decomposition of TBHP. Exothermic onset temperature of 20 mass% aqueous TBHP is lowered from 146.0 ± 1.0 to 138.0 ± 2.0, 133.0 ± 0.0, 97 ± 8.8 and 89 °C by adding 10 mass% DTBP, 20 mass% DTBP, 10 mass% H2O2 and 20 mass% H2O2 relative to the mass of TBHP, respectively. The ranking of solvent effects on the thermal stability of solvated TBHP identified by exothermic onset temperature is arranged from high to low in the following: TBHP in toluene (178.5 ± 1.5 °C) > TBHP in decane (151.0 ± 2.0 °C) > TBHP in H2O (146.0 ± 1.0 °C) > TBHP in nonane (143.5 ± 2.5 °C) > TBHP in Nujol (138.5 ± 0.5 °C) > TBHP in acetone (128.0 ± 2.0 °C) > TBHP in n-BuOH (122.5 ± 3.5 °C).







Similar content being viewed by others
Abbreviations
- A :
-
Frequency factor (s−1 M1−n)
- E a :
-
Activation energy (kJ mol−1)
- ΔH :
-
Heat of reaction (kJ mol−1)
- R:
-
Gas constant (8.314 J mol−1 K−1)
- s :
-
Specific heat (J g−1 K−1)
- T A :
-
Corrected temperature of TBHP formulation under runaway reaction (K)
- T A0 :
-
Corrected onset temperature of TBHP formulation under runaway reaction (K)
- T M :
-
Measured temperature of TBHP formulation under runaway reaction (K)
- T M0 :
-
Measured onset temperature of TBHP formulation under runaway reaction (K)
- dT/dt :
-
Self-heat rate (°C min−1)
- (dT/dt)A(ψ=1) :
-
Self-heat rate corrected to ψ = 1 (°C min−1)
- (dT/dt)M(ψ>1) :
-
Measured self-heat rate at ψ > 1 (°C min−1)
- ΔT AD(ψ=1) :
-
Adiabatic temperature rise corrected to ψ = 1 (K)
- ψ :
-
Thermal inertia
- A:
-
Adiabatic
- AD:
-
Adiabatic
- M:
-
Measured
References
Ho TC, Duh YS, Chen JR. Case studies of incidents in runaway reactions and emergency relief. Process Saf Prog. 1998;17:259–62.
Duh YS, Wu XH, Kao CS. Hazard ratings for organic peroxides. Process Saf Prog. 2008;27:89–99.
Huiping L, Lanyun G, Peng Z, Zhangrui L, Bo Z. Evaluation on the thermal hazard of tert-butyl hydroperoxide by using accelerating rate calorimeter. Proced Eng. 2012;45:574–9.
Hsu JM, Su MS, Huang CY, Duh YS. Calorimetric studies and lessons on fires and explosions of a chemical plant producing CHP and DCPO. J Hazard Mater. 2012;217–218:19–29.
Yeh PY, Shu CM, Duh YS. Thermal hazard analysis of methyl ethyl ketone peroxide. Ind Eng Chem Res. 2002;42:1–5.
Duh YS, Kao CS, Lee C, Yu SW. Runaway hazard assessment of cumene hydroperoxide from the cumene oxidation process. Trans I Chem E Part B Process Saf Environ Prot. 1997;75:79–80.
Duh YS, Kao CS, Hwang HH, Lee WL. Thermal decomposition kinetics of cumene hydroperoxide. Trans I Chem E Part B Process Saf Environ Prot. 1998;76:271–6.
Wang YW, Shu CM, Duh YS, Kao CS. Thermal runaway hazards of cumene hydroperoxide with contaminants. Ind Eng Chem Res. 2001;40:1125–32.
Hou HY, Shu CM, Duh YS. Exothermic decomposition of cumene hydroperoxide at low temperature conditions. AIChE J. 2001;47:1893–6.
Hou HY, Liao TS, Duh YS, Shu CM. Thermal hazard studies for dicumyl peroxide by DSC and TAM. J Therm Anal Calorim. 2006;83:1–5.
Hou HY, Duh YS, Lee WL, Shu CM. Hazard evaluation for redox system of cumene hydroperoxide mixed with inorganic alkaline solutions. J Therm Anal Calorim. 2009;95:541–5.
U.S. Chemical Safety and Hazard Investigation Board. Fire and explosion hazards of benzoyl peroxide. No. 2003-03-C-OH, October 2003.
Recommendations on the transport of dangerous goods, vol. I. 17th revised ed. New York and Geneva: United Nation publication; 2011. p. 105–125.
NFPA 432, Code for the storage of organic peroxide formulations, 2002 ed. National Fire Protection Association, Quincy; 2002
NFPA 704, Standard system for the identification of the hazards materials for emergency response, 2012 ed. National Fire Protection Association, Quincy; 2012.
Recommendation on the transport of dangerous goods, manual of tests and criterion. 5th revised ed. Part II, New York and Geneva: United Nation publication; 2009. p 205–317.
Hiatt R, Clipsham J, Visser T. The induced decomposition of tert-butyl hydroperoxide. Can J Chem. 1964;42:2754–7.
Hiatt R, Mill T, Mayo FR. Homolytic decompositions of hydroperoxides. Ι. Summary and implications for autoxidation. J Org Chem. 1967;33:1416–20.
Hiatt R, Mill T, Irwin KC, Castlman JK. Homolytic decompositions of hydroperoxides. ΙΙ. Radical-induced decompositions of t-butyl hydroperoxide. J Org Chem. 1967;33:1421–7.
Hiatt R, Irwin KC. Homolytic decompositions of hydroperoxides. V. Thermal decompositions of t-butyl hydroperoxide. J Org Chem. 1967;33:1436–41.
Thomas JR. The self-reactions of t-butylperoxy radicals. J Am Chem Soc. 1965;87:3935–40.
Nangia PS, Benson SW. The kinetics of the interaction of peroxy radicals. Ι. The tertiary peroxy radicals. Int J Chem Kinet. 1980;12:29–42.
Wang YW, Duh YS, Shu CM. Characterization of the self-reactive decomposition of tert-butyl hydroperoxide in three different diluents. Process Saf Prog. 2007;26:299–303.
Wang YW, Duh YS, Shu CM. Thermal runaway hazards of tert-butyl hydroperoxide by calorimetric studies. J Therm Anal Calorim. 2009;95:553–7.
ASTM E476-87. Standard test method for thermal instability of confined condensed phase systems (confinement test), American Society for Testing and Materials; 2001.
Townsend DI, Tou JC. Thermal hazard evaluation by an accelerating rate calorimeter. Thermochim Acta. 1980;37:1–30.
Emergency relief system design using DIERS technology: the design institute for emergency relief systems (DIERS) project manual, AIChE, New York; 1992.
Hiatt R. Chapter Ι. Hydroperoxides. In: Swern D, editor. Organic peroxides, vol. II. 2nd ed. New York: Robert E. Krieger publishing company, Inc.; 1981. p. 1–151.
Tse KM. The kinetics and induced decomposition on the thermal decomposition of hydroperoxide. MS thesis. Brock University, St. Catharines, Ontario, Canada. 1976.
Iizuka Y, Surianarayanan M. Comprehensive kinetic model for adiabatic decomposition of di-tert-butyl peroxide using BatchCAD. Ind Eng Chem Res. 2003;42:2987–95.
Kersten RJA, Boers MN, Stork MM, Visser C. Results of a round-robin with di-tertbutyl peroxide in various adiabatic equipment for assessment of runaway reaction hazards. J Loss Prevent Proc. 2005;18:145–51.
Andreozzi R, Caprio V, Crescitelli S, Russo G. The thermal stability of tert-butyl hydroperoxide-acid mixture. J Hazard Mater. 1988;17:305–13.
Wehrstedt KD, Knorr A, Schuurman P. The (MCPVT) as a screening or classification test for explosive properties of organic peroxides. J Loss Prev Ind. 2003;16:523–31.
Shanley ES. Chapter V. Organic peroxides: evaluation and management of hazards. In: Swern D, editor. Organic peroxides, vol. III. 2nd ed. New York: Robert E. Krieger publishing company, Inc.; 1981. p. 341–64.
Acknowledgements
The authors wish to thank National Science Council, R.O.C., for financial support of this study under Contract No. NSC 101-2221-E-239-017-MY3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duh, YS., Kuo, HY. & Kao, CS. Characterization on thermal decompositions of tert-butyl hydroperoxide (TBHP) by confinement test. J Therm Anal Calorim 127, 1047–1059 (2017). https://doi.org/10.1007/s10973-016-5898-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5898-4