Abstract
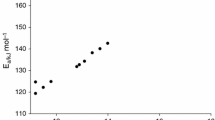
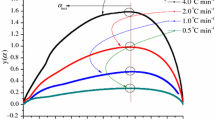
Having two active peroxide groups, 1,1-bis(tert-butylperoxy)cyclohexane (BTBPC) has a certain degree of thermal instability. It is usually used as an initiator in a chemical process, and therefore, careless operation could result in severe accidents. This study emphasized the runaway reactions of BTBPC 70 mass% (4.5–5.2 mg), the relevant thermokinetic parameters, and the thermal safety parameters. Differential scanning calorimetry was used to evaluate the above-mentioned thermokinetic parameters, using four low heating rates (0.5, 1, 2, and 4 °C min−1) combined with kinetic simulation method. The results indicated that apparent exothermic onset temperature (T o), apparent activation energy (E a), and heat of decomposition (ΔH d) were ca. 118 °C, 156 kJ mol−1, and 1,080 kJ kg−1, respectively. In view of process loss prevention, at the low heating rates of 0.5, 1, 2, and 4 °C min−1, storing BTBPC 70 mass% below 27.27 °C is a more reassuring approach.








Similar content being viewed by others
Abbreviations
- A :
-
Pre-exponential factor in the Arrhenius equation (s−1 M1−n)
- C p :
-
Specific heat capacity (J g−1 K−1)
- CV:
-
Coefficient of variation (dimensionless)
- E a :
-
Apparent activation energy of reaction (kJ mol−1)
- ΔH d :
-
Heat of decomposition (kJ kg−1)
- k :
-
Reaction rate constant (mol L−1 s−1)
- k o :
-
Pre-exponential factor of reaction (s−1 M1−n)
- m :
-
Mass of reactant (g)
- n :
-
Order of reaction (dimensionless)
- n i :
-
Reaction order of ith stage (dimensionless)
- r i :
-
Reaction rate of ith stage (g s−1)
- SD:
-
Standard deviation (dimensionless)
- ΔT ad :
-
Adiabatic temperature rise (°C)
- TCL:
-
Time to conversion limit (day)
- TER:
-
Total energy release (kJ kg−1)
- T ext :
-
Extrapolated peak temperature of thermal curve by software of DSC device (°C)
- TMR ad :
-
Time to maximum heating rate under adiabatic conditions (min, h, day)
- T m :
-
Temperature of DSC thermal curve exhibits maximum enthalpy (°C)
- T o :
-
Apparent exothermic onset temperature of decomposition reaction (°C)
- T p :
-
True peak temperature of thermal curve by DSC detector (°C)
- \( \overline{X} \) :
-
Sample mean (variable)
- Z :
-
Autocatalytic constant (variable)
- α :
-
Degree of conversion (dimensionless)
- Β :
-
Heating rate of DSC experiment (°C min−1)
References
Chen JR, Wu SH, Lin SY, Hou HY, Shu CM. Utilization of microcalorimetry for an assessment of the potential for a runaway decomposition of cumene hydroperoxide at low temperatures. J Therm Anal Calorim. 2008;93:127–33.
Chi JH, Wu SH, Shu CM. Thermal explosion analysis of methyl ethyl ketone peroxide by non-isothermal and isothermal calorimetric applications. J Hazard Mater. 2009;171:1145–9.
Chu YC, Tsai FC, Lin FR, Chiang TC, Shu CM. Evaluation unexpected energy released for three liquid organic peroxides. Energy Educ Sci Tech Pt A. 2013;30:977–82.
Lu KT, Chu Y, Chen T, Hu K. Investigation of the decomposition reaction and dust explosion characteristics of crystalline dicumyl peroxide. Process Saf Environ Prot. 2010;88:356–65.
Liu SH, Hou HY, Shu CM. Effects of thermal runaway hazard for three organic peroxides conducted by acids and alkalines with DSC, VSP2, and TAM III. Thermochim Acta. 2013;556:226–32.
Hsueh KH, Chen WT, Chu YC, Tsai LC, Shu CM. Thermal reactive hazards of 1,1-bis(tert-butylperoxy)cyclohexane with nitric acid contaminants by DSC. J Therm Anal Calorim. 2012;109:1253–60.
Li AC, Tsai YT, Wu SH, Chiu CW, Shen SJ, Hsin R, Shu CM. Thermal runaway analysis for two organic peroxides with H2O and dry fire-extinguishing chemicals by DSC and VSP2. J Therm Anal Calorim. 2013;113:1611–8.
Yeh PY, Shu CM, Duh YS. Thermal hazard analysis of methyl ethyl ketone peroxide. Ind Eng Chem Res. 2003;42:1–5.
Chang RH, Shu CM, Duh YS, Jehng JM. Calorimetric studies on the thermal hazard of methyl ethyl ketone peroxide with incompatible substances. J Hazard Mater. 2007;141:762–8.
Kee JK, Kyu YC, Alexander JC. Dynamics of a continuous stirred tank reactor for styrene polymerization initiated by a binary initiator mixture. II: Effect of viscosity dependent heat transfer coefficient. Polym Eng Sci. 1992;32:494–505.
Liu ZZ. Plastic materials—PS market’s present situation. IBP Technology Co., Ltd. http://www.ibuyplastic.com/tech_center/tech_paper/tech_detailcontent_print.phtml?id=576.
Hsu JM, Su MS, Huang CY, Duh YS. Calorimetric studies and lessons on fires and explosions of a chemical plant producing CHP and DCPO. J Hazard Mater. 2012;217–218:19–28.
Tseng JM, Lin CP, Huang ST, Hsu J. Kinetic and safety parameters analysis for 1,1,-di-(tert-butylperoxy)-3,3,5-trimethylcyclohexane in isothermal and non-isothermal conditions. J Hazard Mater. 2011;192:1427–36.
Lin CP, Tseng JM. Green technology for improving process manufacturing design and storage. Chem Eng J. 2012;180:284–92.
Lin CP, Chang CP, Chou YC, Chu YC, Shu CM. Modeling solid thermal explosion containment on reactor HNIW and HMX. J Hazard Mater. 2010;176:549–58.
Kandelbauer A, Wuzella G, Mahendran A, Taudes I, Widsten P. Model-free kinetic analysis of melamine-formaldehyde resin cure. Chem Eng J. 2009;152:556–65.
Kossoy AA, Benin AI, Akhmetshin YG. An advanced approach to reactivity rating. J Hazard Mater. 2005;118:9–17.
ChemicalBook, Inc., Chemcial Product Information, http://www.chemicalbook.com/ProductChemicalPropertiesCB7451603_EN.htm#MSDSA.
Toledo Mettler. STARe Software with Solaris Operating System. Lund: Operating Instructions; 2004.
Lega D, Antonini A, Ciccioli A, Brutti S, Lenzuni P. Low scan rate DSC study of the monoclinic-tetragonal transition in zirconia. Thermochim Acta. 2011;524:18–22.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal. 1970;2:301–24.
Ravi P, Gore GM, Sikder AK, Tewari SP. Thermal decomposition kinetics of 1-methyl-3,4,5-trinitropyrazole. Thermochim Acta. 2012;528:53–7.
ChemInform Saint-Petersburg (CISP) Ltd. Thermal Safety Software (TSS). http://www.cisp.spb.ru/.
Kossoy AA, Akhmetshin Y. Identification of kinetic models for the assessment of reaction hazards. Process Saf Prog. 2007;26:209–20.
Kossoy AA, Sheinman I. Evaluating thermal explosion hazard by using kinetics-based simulation approach. Process Saf Environ Prot. 2004;82:421–30.
Townsend DI, Tou JC. Thermal hazard evaluation by an accelerating rate calorimeter. Thermochim Acta. 1980;37:1–30.
Shangyu Shaofeng Chemical Co., Ltd., http://www.zjsysf.com/pages/pro26.html.
Acknowledgements
The authors wish to express their gratitude to Dr. Arcady A. Kossoy of ChemInform Saint Petersburg (CISP), Ltd., St. Petersburg, Russia, and Mr. Anthony M. Janeshek of Dow Chemical Co., Freeport, Texas, USA, for providing technical assistance. Furthermore, the authors are indebted to Mr. Chia-Yuan Wen for his help on lab tests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsueh, KH., Chen, WC., Liu, SH. et al. Thermal parameters study of 1,1-bis(tert-butylperoxy)cyclohexane at low heating rates with differential scanning calorimetry. J Therm Anal Calorim 118, 1675–1683 (2014). https://doi.org/10.1007/s10973-014-4045-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4045-3