Abstract
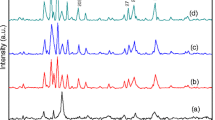
In this work, a comparative study of the application of direct microwave or hydrothermal synthesis for obtaining hydrotalcite from solid initial materials and the effect of these treatments (200 °C, 1–3 h) on the thermal stability of formed compounds were investigated. The molar ratios of primary mixtures Mg/Al were equal to 1 and 2, while the water/solid ratio was maintained to 10.0. It was determined that the microwave treatment was suitable for the direct synthesis of hydrotalcite, although the intensities of diffraction maximums characteristic to this compound were decreased under all experimental conditions. Furthermore, it was examined that, after 3 h of isothermal curing, the highest crystallinity of hydrotalcite was obtained. It should be noted that, in a case of microwave synthesis, the crystallite size of hydrotalcite varied in a 33.89–38.50 nm range, while, in a case of hydrothermal treatment, in a 40.06–52.45 nm region. Moreover, the method used for the synthesis of the latter compound did not influence the formation sequence of compounds during solid sintering (575 and 1000 °C, 1 h). In both cases, at 575 °C temperature, MgO and Al2O3 were formed, while at 1000 °C—magnesium and aluminium spinel-type compounds, which have a similar chemical nature, were obtained. The synthetic and calcined products were characterized by XRD, STA and SEM analyses.






Similar content being viewed by others
References
Sun X, Neuperger E, Dey SK. Insights into the synthesis of layered double hydroxide (LDH) nanoparticles: part 1. Optimization and controlled synthesis of chloride-intercalated LDH. J Colloid Interf Sci. 2015;459:264–72.
Butenko E, Malyshev A, Kapustin A. Influence of hydrocarbon radicals on the structure of layered double hydroxides. Am J Mater Sci Eng. 2014;2:1–6.
Jabłońska M, Chmielarz L, Węgrzyn A, Witkowski S, Michalik M. Mixed metal oxides Mg–Cu–Fe obtained from hydrotalcites as catalysts for selective oxidation of ammonia to nitrogen and water vapour (SCO). Chemik. 2012;66:750–7.
Mrad R, Cousin R, Poupin Ch, Aboukaïs A, Siffert S. Propene oxidation and NO reduction over MgCu–Al(Fe) mixed oxidesderived from hydrotalcite-like compounds. Catal Today. 2015;257:98–103.
Yuan D, Li X, Zhao Q, Zhao J, Tadé M, Liu S. A novel CuTi-containing catalyst derived from hydrotalcite-like compounds for selective catalytic reduction of NO with C3H6 under lean-burn conditions. J Catal. 2014;309:268–79.
Ma W, Meng F, Cheng Z, Sha X, Xin G, Tan D. Synthesized of macroporous composite electrode by activated carbon fiber and Mg–Ca–Al (NO3) hydrotalcite-like compounds to remove bromate. Colloid Surf A. 2015;481:393–9.
Chen Y, Li F, Zhou S, Wei J, Dai Y, Chen Y. Structure and photoluminescence of Mg–Al–Eu ternary hydrotalcite-like layered double hydroxides. J Solid State Chem. 2010;183:2222–6.
Berber MR, Hafez IH, Minagawa K, Katoh M, Mori T, Tanaka M. Uniform nanoparticles of hydrotalcite-like materials and their textural properties at optimized conditions of urea hydrothermal treatment. J Mol Struct. 2013;1033:104–12.
Shi R, Yangn P, Yin Y, Dong X, Li J. Fabrication of porous microspheres and network arraysof Zn–Al hydrotalcite-like compoundson Al substrate via facile hydrothermal method. Ceram Int. 2014;40:6855–63.
Kang HT, Lv K, Yuan SL. Synthesis, characterization, and SO2 removal capacity of MnMgAlFe mixed oxides derived from hydrotalcite-like compounds. Appl Clay Sci. 2013;72:184–90.
Arco M, Gutie´rrez S, Martı´n M, Rives V, Rocha J. Synthesis and characterization of layered double hydroxides (LDH) intercalated with non-steroidal anti-inflammatory drugs (NSAID). J Solid State Chem. 2004;177:3954–62.
Wang Z, Wang E, Gao L, Xu L. Synthesis and properties of Mg2Al layered double hydroxides containing 5-fluorouracil. J Solid State Chem. 2005;178:736–41.
Zhang Z, Chen G, Xu K. One-pot green hydrothermal synthesis of stearate-intercalated MgAl layered double hydroxides. Appl Clay Sci. 2013;72:206–10.
Gao P, Xie R, Wang H, Zhong L, Xi L, Zhang Z, Wei W, Sun Y. Cu/Zn/Al/Zr catalysts via phase-pure hydrotalcite-like compounds for methanol synthesis from carbon dioxide. J CO2 Util. 2015;11:41–8.
Chubar N, Gerda V, Megantari O, Micˇušík M, Omastova M, Heister K, Manf P, Fraissard J. Applications versus properties of Mg–Al layered double hydroxides provided by their syntheses methods: alkoxide and alkoxide-free sol–gel syntheses and hydrothermal precipitation. Chem Eng J. 2013;234:284–99.
Hora L, Kikhtyanin O, Čapek L, Bortnovskiy O, Kubičk D. Comparative study of physico-chemical properties of laboratory and industrially prepared layered double hydroxides and their behavior in aldol condensation of furfural and acetone. Catal Today. 2015;241:221–30.
Ballarini A, Benito P, Fornasari G. Role of the composition and preparation method in the activity of hydrotalcite-derived Ru catalysts in the catalytic partial oxidation of methane. Int J Hydrogen Energy. 2013;38:15128–39.
Scelza O, Vaccari A. Effect of post-synthesis microwave-hydrothermal treatment on the properties of layered double hydroxides and related materials. Appl Clay Sci. 2010;48:218–27.
Liao L, Zhao N, Xia Z. Hydrothermal synthesis of Mg–Al layered double hydroxides (LDHs) from natural brucite and Al(OH)3. Mater Res Bull. 2012;47:3897–901.
Zhang F, Dub N, Li H, Liu J, Hou W. Synthesis of Mg–Al–Fe–NO3 layered double hydroxides via a mechano-hydrothermal route. Solid State Sci. 2014;32:41–7.
Rivera JA, Fetter G, Bosch P. Microwave power effect on hydrotalcite synthesis. Micropor Mesopor Mat. 2006;89:306–14.
Benito P, Guinea I, Labajos FM, Rives V. Microwave-assisted reconstruction of Ni, Al hydrotalcite-like compounds. J Solid State Chem. 2008;181:987–96.
Kloprogge JT, Hickey L, Frost RL. The effect of varying synthesis conditions on zinc chromium hydrotalcite: a spectroscopic study. Mater Chem Phys. 2005;89:99–109.
Kloprogge JT, Hickey L, Frost RL. The effects of synthesis pH and hydrothermal treatment on the formation of zinc aluminum hydrotalcites. J Solid State Chem. 2004;177:4047–57.
Wiyantoko B, Kurniawati P, Purbaningtias TE, Fatimah I. Synthesis and characterization of hydrotalcite at different Mg/Al molar ratios (3rd international seminar on chemistry 2014). Proc Chem. 2015;17:21–6.
Wang L, Li B, Chen Ch, Jia L. Structural characterization and related properties of the stearate anions intercalated Ni–Al hydrotalcite-like compound prepared by the microwave crystallization. J Alloy Compd. 2010;508:426–32.
Benito P, Guinea I, Labajos FM, Rocha J, Rives V. Microwave-hydrothermally aged Zn, Al hydrotalcite-like compounds: influence of the composition and the irradiation conditions. Micropor Mesopor Mat. 2008;110:292–302.
Bergadà O, Vicente I, Salagre P, Cesteros Y, Medina F, Sueiras JE. Microwave effect during aging on the porosity and basic properties of hydrotalcites. Micropor Mesopor Mat. 2007;101:363–73.
Álvarez A, Trujillano R, Rives V. Differently aged gallium-containing layered double hydroxides. Appl Clay Sci. 2013;80–81:326–33.
Benito P, Herrero M, Labajos FM, Rives V. Effect of post-synthesis microwave–hydrothermal treatment on the properties of layered double hydroxides and related materials. Appl Clay Sci. 2010;48:218–27.
Othman MR, Helwani Z, Martunus F, Fernando WJN. Synthetic hydrotalcites from different routes and their application as catalysts and gas adsorbents: a review. Appl Organomet Chem. 2009;23:335–46.
Budhysutanto WN, Kramer HJM, van Agterveld D, Talma AG, Jansens PJ. Pre-treatment of raw materials for the hydrothermal synthesis of hydrotalcite-like compounds. Chem Eng Res Des. 2010;88:1445–9.
Carmo WR, Fischer-Haddad J, Chagas LH, Beltrão MSS, Carvalho GSG, Oliveira LCA, Souza TE, Leitão AA, Diniz R. Effect of precursor synthesis on the physicochemical properties of Zn–Mg–Al mixed oxides. Appl Clay Sci. 2015;116–117:31–8.
Jabłońska M, Chmielarz L, Węgrzyn A, Guzik K, Piwowarska Z, Witkowski S, Walton RI, Dunne PW, Kovanda F. Thermal transformations of Cu–Mg (Zn)–Al(Fe) hydrotalcite-like materials into metal oxide systems and their catalytic activity in selective oxidation of ammonia to dinitrogen. J Therm Anal Calorim. 2013;114:731–47.
Bankauskaitė A, Baltakys K, Mezinskis G. Modified hydrotalcites application as precursors for (Na, K)Mg/Al spinel-type compounds formation. J Therm Anal Calorim. 2014;118:711–8.
Meloni D, Monaci R, Cutrufello MG, Rombi E, Ferino I. Adsorption microcalorimetry characterization of K-doped MgAl mixed oxide catalysts for soybean oil transesterification synthesized by impregnation and ball milling techniques. J Therm Anal Calorim. 2015;119:1023–36.
Bankauskaite A, Baltakys K. Thermal, texture and reconstruction properties of hydrotalcites substituted with Na+ or K+ ions. J Therm Anal Calorim. 2015;121:227–33.
Holgado PH, Holgado MJ, San Román MS, Rives V. Ni–Fe mixed oxides prepared by calcination of layered double hydroxides: potential pigments for the ceramic industry. Ceram Int. 2015;4:8451–60.
Zhang Z, Liao M, Zeng HY, Xu S, Liu X, Du J, Zhu P, Huang QJ. Temperature effect on chromium(VI) removal by Mg/Al mixed metal oxides as adsorbents. Appl Clay Sci. 2014;102:246–53.
Kocík J, Hájek M, Troppová I. The factors influencing stability of Ca–Al mixed oxides as a possible catalyst for biodiesel production. Fuel Process Technol. 2015;134:297–302.
Kloprogge JT, Ruan HD, Frost RL. Thermal decomposition of bauxite minerals: infrared emission spectroscopy of gibbsite, boehmite and diaspore. J Mater Sci. 2002;37:1121–9.
Acknowledgements
This research was funded by a Grant (No. MTEPI- P-15045) from Kaunas University of Technology priority-inter-field and transdisciplinary research projects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zadaviciute, S., Baltakys, K. & Bankauskaite, A. The effect of microwave and hydrothermal treatments on the properties of hydrotalcite. J Therm Anal Calorim 127, 189–196 (2017). https://doi.org/10.1007/s10973-016-5593-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5593-5