Abstract
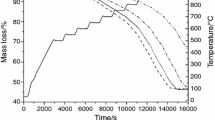
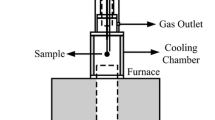
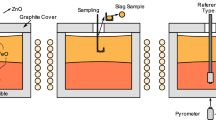
Solid waste generation is one of the main problems in the steelmaking process. One of the most problematic waste products is the electric arc furnace dust, which is a by-product rich in iron and zinc and is present as zincite (zinc oxide) or franklinite (zinc ferrite). This work focuses on the reduction kinetics of synthetic zinc ferrite by gases containing hydrogen and carbon monoxide. This process was examined via forced stepwise isothermal analysis. The test was conducted at temperatures ranging from 500 to 950 °C. Reduction of zinc was accomplished using a mixture of hydrogen and carbon monoxide in order to simulate reformed natural gas. The results indicated that reduction of zinc ferrite occurred in two stages (550–750 °C and 800–900 °C). The first stage was characterized by iron oxide reduction, where a mix control between nucleation and diffusion was determined. The apparent activation energy obtained was 71.5 kJ mol−1. The second stage was characterized by zinc oxide reduction, where the controlling mechanism was identified as a mixed control between diffusion and phase boundary reaction. The apparent activation energy was 135.5 kJ mol−1. The formation of a dense layer of metallic iron around the unreacted core may have caused the apparent activation energy to increase.













Similar content being viewed by others
References
Vieira CMF, Andrade PM, Maciel GS, Vernilli F Jr, Monteiro SN. Incorporation of fine steel sludge waste into red ceramic. Mater Sci Eng A. 2006;427:142–7.
Guézennec AG, Huber JC, Patisson F, Sessiecq P, Birat JP, Ablitze D. Dust formation by bubble-burst phenomenon at the surface of a liquid steel bath. ISIJ Int. 2004;44:1328–33.
Jarupisitthorn C, Pimtong T, Lothongkum G. Investigation of kinetics of zinc leaching from electric arc furnace dust by sodium hydroxide. Mater Chem Phys. 2002;77:531–5.
Martins FM, Neto JMR, Cunha CJ. Mineral phases of weathered and recent electric arc furnace dust. J Hazard Mater. 2008;154:417–25.
Soilic T, Mioc AR, Stefanovic SC, Radovic VN, Jenko M. Characterization of steel mill electric-arc furnace dust. J Hazard Mater B. 2004;109:59–70.
Olivier A, Vegliò F. Process simulation of natural gas steam reforming: fuel distribution optimisation in the furnace. Fuel Process Technol. 2008;89:622–32.
Izquierdo U, Barrio VL, Cambra JF, Requies J, Güemez MB, Arias PL, Kolb G, Zapf R, Gutiérrez AM, Arraibi JR. Hydrogen production from methane and natural gas steam reforming in conventional and microreactor reaction systems. Int J Hydrog Energy. 2012;37:7026–33.
Saito M, Kojima J, Iwai H, Yoshida H. The limiting process in steam methane reforming with gas diffusion into a porous catalytic wall in a flow reactor. Int J Hydrog Energy. 2015;40:8844–55.
Menad N, Ayala JN, Carcedo FG, Hernández A. Study of the presence of fluorine in the recycled fractions during carbothermal treatment of EAF dust. Waste Manag. 2003;23:483–91.
Tong LF, Hayes P. Mechanisms of the reduction of zinc Ferrites in H2/N2 gas mixtures. Miner Process Extract Metall Rev. 2007;28:127–57.
Liang M, Kang W, Xie K. Comparison of reduction behavior of Fe2O3, ZnO and ZnFe2O4 by TPR technique. J Nat Gas Chem. 2009;18:110–3.
Tong LF. Reduction mechanisms and behaviour of zinc ferrite-Part 1: pure ZnFe2O4. Miner Process Extr Metall. 2001;110:14–24.
Lee JJ, Lin CI, Chen HK. Carbothermal reduction of zinc ferrite. Mater Trans B. 2001;32B:1033–40.
Gioia F, Mura G, Viola A. Experimental study of the direct reduction of sinterized zinc oxide by hydrogen. Chem Eng Sci. 1977;32:1401–9.
González G, Jordens ZS, Escobedo S. Dependence of zinc oxide reduction rate on the CO concentration in CO/CO2 mixtures. Thermochim Acta. 1996;278:129–34.
Lew S, Sarofim AF, Flytzani-Stephanopoulos M. The reduction of zinc titanate and zinc oxide solids. Chem Eng Sci. 1992;47:1421–31.
Guger CE, Manning FS. Kinetics of zinc oxide reduction with carbon monoxide. Metall Trans. 1971;2:3083–90.
Zhang YB, Su ZJ, Zhou YL, Li GH, Jiang T. Reduction kinetics of SnO2 and ZnO in the tin, zinc-bearing iron ore pellet under a 20 %CO–80 %CO2 atmosphere. Int J Miner Process. 2013;124:15–9.
Kim BS, Yoo JM, Park JT, Lee JC. A kinetic study of the carbothermic reduction of zinc oxide with various additives. Mater Trans. 2006;47:2421–6.
Zhang B, Yan XY, Shibata K, Uda T, Tada M, Hirasawa M. Thermogravimetric-mass spectrometric analysis of the reaction between oxides (ZnO, Fe2O3 or ZnFe2O4) and polyvinyl chloride under inert atmosphere. Mater Trans JIM. 2000;41:1342–50.
Junca E, Restivo TAG, Espinosa DCR, Tenório JAS. Application of stepwise isothermal analysis method in the kinetic study of reduction of basic oxygen furnace dust. J Therm Anal Calorim. 2015;120:1913–9.
Chen F, Sorensen OT, Meng G, Peng D. Thermal decomposition of BaC2O4·0.5 H2O studied by stepwise isothermal analysis and non-isothermal thermogravimetry. J Therm Anal. 1998;53:397–410.
Maqueda LAP, Ortega A, Criado JM. The use of master plots for discriminating the kinetic model of solid state reactions from a single constant-rate thermal analysis (CRTA) experiment. Thermochim Acta. 1996;277:165–73.
Yu D, Zhu M, Utigard TA, Barati M. TG/DTA study on the carbon monoxide and graphite thermal reduction of a high-grade iron nickel oxide residue with the presence of siliceous gangue. Themochim Acta. 2014;575:1–11.
Focht GD, Ranade PV, Harrison DP. High-temperature desulfurization using zinc ferrite: reduction and sulfidation kinetics. Chem Eng Sci. 1988;43:3005–13.
Lina HY, Chena YW, Li C. The mechanism of reduction of iron oxide by hydrogen. Thermochim Acta. 2003;400:61–7.
Yu D, Zhu M, Utigard TA, Barati M. TGA kinetic study on the hydrogen reduction of an iron nickel oxide. Miner Eng. 2013;54:32–8.
Moukassi M, Steinmetz P, Dupre B, Gleitzer C. Mechanism of reduction with hydrogen of pure wustite single crystals. Metall Trans B Process Metall. 1983;14B:125–32.
Wiltowski T, Piotrowski K, Lorethova H, Stonawski L, Mondal K, Lalvani SB. Neural network approximation of iron oxide reduction process. Chem Eng Process Process Intensif. 2005;44:775–83.
Acknowledgements
The authors would like to thank FAPESP (the State of São Paulo’s Research Support Foundation) process 11/51638-0, the CNPq (National Council for Scientific and Technological Development) process 245470/2012-3, the CAPES (Coordination for the Improvement of Higher Education Personnel) Foundation, Project PE003/2008, the University of São Paulo and FAPES (the State of Espírito Santo’s Research and innovation Support Foundation), Process 68853777/14.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Junca, E., de Oliveira, J.R., Restivo, T.A.G. et al. Synthetic zinc ferrite reduction by means of mixtures containing hydrogen and carbon monoxide. J Therm Anal Calorim 123, 631–641 (2016). https://doi.org/10.1007/s10973-015-4973-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4973-6